Synthesis and spectral characterization of a long-lifetime osmium (II) metal-ligand complex: a conjugatable red dye for applications in biophysics
- PMID: 10483708
- PMCID: PMC6901021
- DOI: 10.1016/s0301-4622(99)00069-1
Synthesis and spectral characterization of a long-lifetime osmium (II) metal-ligand complex: a conjugatable red dye for applications in biophysics
Abstract
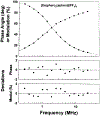
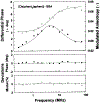
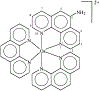
There is a need for luminescent probes, which display both long excitation and emission wavelengths and long decay times. We synthesized and characterized an osmium metal-ligand complex which displays a mean decay time of over 100 ns when bound to proteins. [Os(1,10-phenanthroline)2(5-amino-1,10-phenanthroline)[(PF6)2 can be excited at wavelengths up to 650 nm, and displays an emission maximum near 700 nm. The probe displays a modest but useful maximum fundamental anisotropy near 0.1 for 488-nm excitation, and thus convenient when using an argon ion laser. [Os(phen)2(aphen)](PF6)2 is readily activated to the isothiocyanate for coupling to proteins. When covalently linked to bovine serum albumin the intensity decay is moderately heterogeneous with a mean decay time of 145 ns. The anisotropy decay of the labeled protein displays a correlation time near 40 ns. This relatively long lifetime luminophores can be useful as a biophysical probe or in clinical applications such as fluorescence polarization immunoassays.
Figures



Similar articles
-
Synthesis and spectral characterization of a thiol-reactive long-lifetime Ru(II) complex.Anal Biochem. 1997 Sep 5;251(2):241-5. doi: 10.1006/abio.1997.2253. Anal Biochem. 1997. PMID: 9299022 Free PMC article.
-
[Os(bpy)2(CO)(enIA)][OTf]2: a novel sulfhydryl-specific metal-ligand complex.Inorg Chem. 2005 May 30;44(11):3875-9. doi: 10.1021/ic048452q. Inorg Chem. 2005. PMID: 15907113
-
A long-lived, highly luminescent Re(I) metal-ligand complex as a biomolecular probe.Anal Biochem. 1997 Dec 15;254(2):179-86. doi: 10.1006/abio.1997.2413. Anal Biochem. 1997. PMID: 9417774 Free PMC article.
-
Mononuclear and dinuclear complexes of dibenzoeilatin: synthesis, structure, and electrochemical and photophysical properties.Inorg Chem. 2004 Apr 5;43(7):2355-67. doi: 10.1021/ic0354062. Inorg Chem. 2004. PMID: 15046512
-
Photogenerated avenues in macromolecules containing Re(I), Ru(II), Os(II), and Ir(III) metal complexes of pyridine-based ligands.Chem Soc Rev. 2012 Mar 21;41(6):2222-55. doi: 10.1039/c1cs15154a. Epub 2011 Nov 14. Chem Soc Rev. 2012. PMID: 22080248 Review.
Cited by
-
Electron Transfer in Nitrogenase.Chem Rev. 2020 Jun 24;120(12):5158-5193. doi: 10.1021/acs.chemrev.9b00663. Epub 2020 Jan 30. Chem Rev. 2020. PMID: 31999100 Free PMC article. Review.
-
Dynamics of bacteriophage R17 probed with a long-lifetime Ru(II) metal-ligand complex.J Fluoresc. 2010 May;20(3):713-8. doi: 10.1007/s10895-010-0612-6. Epub 2010 Feb 27. J Fluoresc. 2010. PMID: 20195712
-
Fluorescence lifetime measurements and biological imaging.Chem Rev. 2010 May 12;110(5):2641-84. doi: 10.1021/cr900343z. Chem Rev. 2010. PMID: 20356094 Free PMC article. Review. No abstract available.
-
High-molecular-weight protein hydrodynamics studied with a long-lifetime metal-ligand complex.Biochim Biophys Acta. 2002 Jun 3;1597(2):221-8. doi: 10.1016/s0167-4838(02)00281-9. Biochim Biophys Acta. 2002. PMID: 12044900 Free PMC article.
References
-
- Daehne S, Resch-Genger U, Wolfbeis OS, Near-infrared dyes for high technology applications, Proceedings of the NATO Advanced Research Workshop on Syntheses, Optical Properties and Applications of Near-Infrared (NIR) Dyes in High Technology, Czech Republic, Kluwer Acad. Publishers, 1998, pp. 468.
-
- Casay GA, Shealy DB, Patonay G, Near-infrared fluorescence probes, in: Lakowicz JR(Ed.), Topics in Fluorescence Spectroscopy, Probe Design and Chemical Sensing, vol. 4, Plenum Press, New York, 1994, pp. 183–222.
-
- Patonay G, Antonie MD, Near-infrared fluorogenic labels: New approach to an old problem, Anal. Chem 63 (1991) 321A–327A.
-
- Thompson RB, Red and near-infrared fluorometry, in: Lakowicz JR (Ed.), Topics in Fluorescence Spectroscopy, Probe Design and Chemical Sensing, vol. 4, Plenum Press, New York, 1994, pp. 151–181.
-
- Miller JN, Brown MB, Seare NJ, Summerfield S, Analytical applications of very near-IR fluorimetry, in: Wolfbeis OS (Ed.), Fluorescence Spectroscopy, Springer-Verlag, New York, 1993, pp. 189–196.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

