A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development
- PMID: 10485845
- PMCID: PMC316998
- DOI: 10.1101/gad.13.17.2218
A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development
Abstract
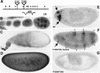
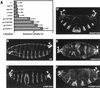
The Drosophila gene groucho (gro) encodes a transcriptional corepressor that has critical roles in many development processes. In an effort to illuminate the mechanism of Gro-mediated repression, we have employed Gro as an affinity reagent to purify Gro-binding proteins from embryonic nuclear extracts. One of these proteins was found to be the histone deacetylase Rpd3. Protein-protein interaction assays suggest that Gro and Rpd3 form a complex in vivo and that they interact directly via the glycine/proline rich (GP) domain in Gro. Cell culture assays demonstrate that Rpd3 potentiates repression by the GP domain. Furthermore, experiments employing a histone deacetylase inhibitor, as well as a catalytically inactive form of Rpd3, imply that histone deacetylase activity is required for efficient Gro-mediated repression. Finally, mutations in gro and rpd3 have synergistic effects on embryonic lethality and pattern formation. These findings support the view that Gro mediates repression, at least in part, by the direct recruitment of the histone deacetylase Rpd3 to the template, where it can modulate local chromatin structure. They also provide evidence for a specific role of Rpd3 in early development.
Figures








References
-
- Belikov S, Karpov V. Linker histones: Paradigm lost but questions remain. FEBS Lett. 1998;441:161–164. - PubMed
-
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes & Dev. 1993;7:592–604. - PubMed
-
- Campos-Ortega JA, Hartenstein V. The embyronic development of Drosophila melanogaster. Berlin, Germany: Springer-Verlag; 1985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
