Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation
- PMID: 10485846
- PMCID: PMC316997
- DOI: 10.1101/gad.13.17.2231
Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation
Abstract
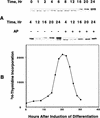
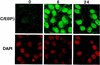
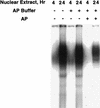
Hormonal induction of 3T3-L1 preadipocytes triggers a cascade of events that initiate differentiation into adipocytes. CCAAT/enhancer-binding proteins beta and delta (C/EBPbeta/delta) are expressed early in the differentiation program, but are not immediately active. After a long lag, C/EBPbeta/delta become competent to bind to the C/EBP regulatory element in the C/EBPalpha gene promoter, C/EBPalpha being a transcriptional activator of numerous adipocyte genes. As C/EBPbeta/delta acquire binding activity, they become localized to centromeres as preadipocytes synchronously enter S phase at the onset of mitotic clonal expansion. Localization to centromeres occurs through C/EBP consensus-binding sites in centromeric satellite DNA. C/EBPalpha, which is antimitotic, becomes centromere-associated much later in the differentiation program as mitotic clonal expansion ceases and the cells become terminally differentiated.
Figures
















References
-
- Baer M, Williams SC, Dillner A, Schwartz RC, Johnson PF. Autocrine signals control CCAAT/enhancer binding protein β expression, localization and activity in macrophages. Blood. 1998;92:4353–4365. - PubMed
-
- Bernlohr DA, Bolanowski MA, Kelly TJ, Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985;260:5563–5567. - PubMed
-
- Brun RP, Tontonoz P, Forman BM, Ellis R, Jasmine C, Evans RM, Spiegelman BM. Differential activation of adipogensis by multiple PPAR isoforms. Genes & Dev. 1996;10:974–984. - PubMed
-
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes & Dev. 1991;5:1538–1552. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
