Nuclear export of Far1p in response to pheromones requires the export receptor Msn5p/Ste21p
- PMID: 10485850
- PMCID: PMC317000
- DOI: 10.1101/gad.13.17.2284
Nuclear export of Far1p in response to pheromones requires the export receptor Msn5p/Ste21p
Abstract
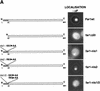
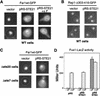
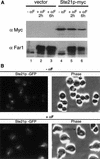
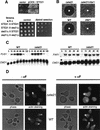
Far1p is a bifunctional protein that is required to arrest the cell cycle and to establish cell polarity during yeast mating. Far1p is localized predominantly in the nucleus but accumulates in the cytoplasm in cells exposed to pheromones. Here we show that Far1p functions in both subcellular compartments: nuclear Far1p is required to arrest the cell cycle, whereas cytoplasmic Far1p is involved in the establishment of cell polarity. The subcellular localization of Far1p is regulated by two mechanisms: (1) Far1p contains a functional bipartite nuclear localization signal (NLS), and (2) Far1p is exported from the nucleus by Msn5p/Ste21p, a member of the exportin family. Cells deleted for Msn5p/Ste21p failed to export Far1p in response to pheromones, whereas overexpression of Msn5p/Ste21p was sufficient to accumulate Far1p in the cytoplasm in the absence of pheromones. Msn5p/Ste21p was localized in the nucleus and interacted with Far1p in a manner dependent on GTP-bound Gsp1p. Two-hybrid analysis identified a small fragment within Far1p that is necessary and sufficient for binding to Msn5p/Ste21p, and is also required to export Far1p in vivo. Finally, similar to Deltamsn5/ste21 strains, cells expressing a mutant Far1p, which can no longer be exported, exhibit a mating defect, but are able to arrest their cell cycle in response to pheromones. Taken together, our results suggest that nuclear export of Far1p by Msn5p/Ste21p coordinates the two separable functions of Far1p during mating.
Figures









References
-
- Arkowitz RA. Responding to attraction: Chemotaxis and chemotropism in Dictyostelium and yeast. Trends Cell Biol. 1999;9:20–27. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: Greene Publishing Associates and Wiley-Interscience; 1991.
-
- Bähler J, Peter M. Cell polarity in yeast. In: Drubin DG, editor. Frontiers in molecular biology: cell polarity. Oxford University Press; 1999. (In press).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
