The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs
- PMID: 10485852
- PMCID: PMC316995
- DOI: 10.1101/gad.13.17.2315
The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs
Abstract
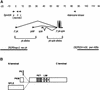
The tissue polarity mutants in Drosophila include a set of conserved gene products that appear to be involved in the control of cytoskeletal architecture. Here we show that the tissue polarity gene prickle (pk) encodes a protein with a triple LIM domain and a novel domain that is present in human, murine, and Caenorhabditis elegans homologs which we designate PET. Three transcripts have been identified, pk, pkM, and sple, encoding 93-, 100-, and 129-kD conceptual proteins, respectively. The three transcripts span 70 kb and share 6 exons that contain the conserved domains. The pk and sple transcripts are expressed with similar tissue-specific patterns but have qualitatively different activities. The phenotypes of pk mutants, and transgenic flies in which the different isoforms are overexpressed show that the balance between Pk and Sple is critical for the specification of planar polarity. In addition, these phenotypes suggest a tessellation model in which the alignment of wing hairs is dependent on cell shape and need not reflect fine-grained positional information. Lack of both pk and sple transcripts gives a phenotype affecting the whole body surface that is similar to those of dishevelled and frizzled (fz) suggesting a functional relationship between pk and fz signaling.
Figures



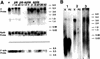




References
-
- Adler PN. The genetic control of tissue polarity in Drosophila. BioEssays. 1992;14:735–741. - PubMed
-
- Ashburner M. Drosophila, a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
-
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S. Interaction between Wingless and Notch signaling pathways mediated by Dishevelled. Science. 1996;271:1826–1832. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
