Evidence for the function of an exonic splicing enhancer after the first catalytic step of pre-mRNA splicing
- PMID: 10485881
- PMCID: PMC17938
- DOI: 10.1073/pnas.96.19.10655
Evidence for the function of an exonic splicing enhancer after the first catalytic step of pre-mRNA splicing
Abstract
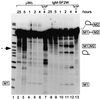
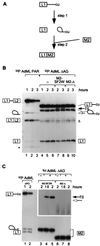
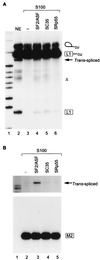
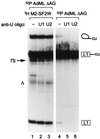
Exonic splicing enhancers (ESEs) activate pre-mRNA splicing by promoting the use of the flanking splice sites. They are recognized by members of the serine/arginine-rich (SR) family of proteins, such as splicing factor 2/alternative splicing factor (SF2/ASF), which recruit basal splicing factors to form the initial complexes during spliceosome assembly. The in vitro splicing kinetics of an ESE-dependent IgM pre-mRNA suggested that an SF2/ASF-specific ESE has additional functions later in the splicing reaction, after the completion of the first catalytic step. A bimolecular exon ligation assay, which physically uncouples the first and second catalytic steps of splicing in a trans-splicing reaction, was adapted to test the function of the ESE after the first step. A 3' exon containing the SF2/ASF-specific ESE underwent bimolecular exon ligation, whereas 3' exons without the ESE or with control sequences did not. The ESE-dependent trans-splicing reaction occurred after inactivation of U1 or U2 small nuclear ribonucleoprotein particles, compatible with a functional assay for events after the first step of splicing. The ESE-dependent step appears to take place before the ATP-independent part of the second catalytic step. Bimolecular exon ligation also occurred in an S100 cytosolic extract, requiring both the SF2/ASF-dependent ESE and complementation with SF2/ASF. These data suggest that some ESEs can act late in the splicing reaction, together with appropriate SR proteins, to enhance the second catalytic step of splicing.
Figures


References
-
- Cáceres J F, Krainer A R. In: Eukaryotic mRNA Processing. Krainer A R, editor. Oxford: IRL Press; 1997. pp. 174–212.
-
- Alzhanova-Ericsson A T, Sun X, Visa N, Kiseleva E, Wurtz T, Daneholt B. Genes Dev. 1996;10:2881–2893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

