Toward the bilayer proteome, electrospray ionization-mass spectrometry of large, intact transmembrane proteins
- PMID: 10485888
- PMCID: PMC17945
- DOI: 10.1073/pnas.96.19.10695
Toward the bilayer proteome, electrospray ionization-mass spectrometry of large, intact transmembrane proteins
Abstract
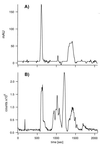
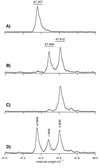
Genes encoding membrane proteins comprise a substantial proportion of genomes sequenced to date, but ability to perform structural studies on this portion of the proteome is limited. Electrospray ionization-MS (ESI-MS) of an intact protein generates a profile defining the native covalent state of the gene product and its heterogeneity. Here we apply ESI-MS technology with accuracy exceeding 0.01% to a hydrophobic membrane protein with 12-transmembrane alpha-helices, the full-length lactose permease from Escherichia coli. Furthermore, ESI-MS is used to titrate reactive thiols with N-ethylmaleimide. Treatment of the native protein solubilized in detergent micelles reveals only two reactive thiols, and both are protected by a substrate analog.
Figures

Similar articles
-
Proteomics on full-length membrane proteins using mass spectrometry.Biochemistry. 2000 Apr 18;39(15):4237-42. doi: 10.1021/bi000150m. Biochemistry. 2000. PMID: 10757971
-
Elucidation of substrate binding interactions in a membrane transport protein by mass spectrometry.EMBO J. 2003 Apr 1;22(7):1467-77. doi: 10.1093/emboj/cdg145. EMBO J. 2003. PMID: 12660154 Free PMC article.
-
Cysteine-scanning mutagenesis of helix II and flanking hydrophilic domains in the lactose permease of Escherichia coli.Biochemistry. 1997 Jan 7;36(1):269-73. doi: 10.1021/bi9618629. Biochemistry. 1997. PMID: 8993343
-
The lactose permease of Escherichia coli: a paradigm for membrane transport proteins.Soc Gen Physiol Ser. 1993;48:1-9. Soc Gen Physiol Ser. 1993. PMID: 8503038 Review. No abstract available.
-
Structural comparison of lactose permease and the glycerol-3-phosphate antiporter: members of the major facilitator superfamily.Curr Opin Struct Biol. 2004 Aug;14(4):413-9. doi: 10.1016/j.sbi.2004.07.005. Curr Opin Struct Biol. 2004. PMID: 15313234 Review.
Cited by
-
Hydrophobic Proteome Analysis of Triple Negative and Hormone-Receptor-Positive-Her2-Negative Breast Cancer by Mass Spectrometer.Clin Proteomics. 2010 Sep;6(3):93-103. doi: 10.1007/s12014-010-9052-1. Epub 2010 Sep 7. Clin Proteomics. 2010. PMID: 20930921 Free PMC article.
-
Best practices and benchmarks for intact protein analysis for top-down mass spectrometry.Nat Methods. 2019 Jul;16(7):587-594. doi: 10.1038/s41592-019-0457-0. Epub 2019 Jun 27. Nat Methods. 2019. PMID: 31249407 Free PMC article. Review.
-
Evolutionary migration of a post-translationally modified active-site residue in the proton-pumping heme-copper oxygen reductases.Biochemistry. 2006 Dec 26;45(51):15405-10. doi: 10.1021/bi062026u. Epub 2006 Dec 19. Biochemistry. 2006. PMID: 17176062 Free PMC article.
-
Analysis of a G protein-coupled receptor for neurotensin by liquid chromatography-electrospray ionization-mass spectrometry.Anal Biochem. 2008 May 1;376(1):13-24. doi: 10.1016/j.ab.2007.12.025. Epub 2007 Dec 27. Anal Biochem. 2008. PMID: 18294946 Free PMC article.
-
Kinetic isotope effect reveals rate-limiting step in green-to-red photoconvertible fluorescent proteins.Protein Sci. 2024 Jul;33(7):e5069. doi: 10.1002/pro.5069. Protein Sci. 2024. PMID: 38864740 Free PMC article.
References
-
- Lander E S. Science. 1996;274:536–539. - PubMed
-
- Ashton D S, Beddell C R, Green B N, Oliver R W. FEBS Lett. 1994;342:1–6. - PubMed
-
- Fenn J B, Mann M, Meng C K, Wong S F, Whitehouse C M. Science. 1989;246:64–71. - PubMed
-
- Covey T R, Bonner R F, Shushan B I, Henion J. Rapid Commun Mass Spectrom. 1988;2:249–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

