Muscle degeneration without mechanical injury in sarcoglycan deficiency
- PMID: 10485893
- PMCID: PMC17950
- DOI: 10.1073/pnas.96.19.10723
Muscle degeneration without mechanical injury in sarcoglycan deficiency
Abstract
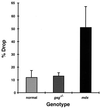
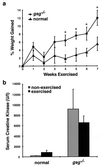
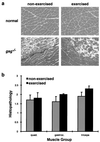
In humans, mutations in the genes encoding components of the dystrophin-glycoprotein complex cause muscular dystrophy. Specifically, primary mutations in the genes encoding alpha-, beta-, gamma-, and delta-sarcoglycan have been identified in humans with limb-girdle muscular dystrophy. Mice lacking gamma-sarcoglycan develop progressive muscular dystrophy similar to human muscular dystrophy. Without gamma-sarcoglycan, beta- and delta-sarcoglycan are unstable at the muscle membrane and alpha-sarcoglycan is severely reduced. The expression and localization of dystrophin, dystroglycan, and laminin-alpha2, a mechanical link between the actin cytoskeleton and the extracellular matrix, appears unaffected by the loss of sarcoglycan. We assessed the functional integrity of this mechanical link and found that isolated muscles lacking gamma-sarcoglycan showed normal resistance to mechanical strain induced by eccentric muscle contraction. Sarcoglycan-deficient muscles also showed normal peak isometric and tetanic force generation. Furthermore, there was no evidence for contraction-induced injury in mice lacking gamma-sarcoglycan that were subjected to an extended, rigorous exercise regimen. These data demonstrate that mechanical weakness and contraction-induced muscle injury are not required for muscle degeneration and the dystrophic process. Thus, a nonmechanical mechanism, perhaps involving some unknown signaling function, likely is responsible for muscular dystrophy where sarcoglycan is deficient.
Figures

Similar articles
-
Sarcoglycans in muscular dystrophy.Microsc Res Tech. 2000 Feb 1-15;48(3-4):167-80. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<167::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10679964 Review.
-
Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex.J Cell Sci. 2000 Jul;113 ( Pt 14):2535-44. doi: 10.1242/jcs.113.14.2535. J Cell Sci. 2000. PMID: 10862711
-
Extraocular muscle is spared despite the absence of an intact sarcoglycan complex in gamma- or delta-sarcoglycan-deficient mice.Neuromuscul Disord. 2001 Mar;11(2):197-207. doi: 10.1016/s0960-8966(00)00171-1. Neuromuscul Disord. 2001. PMID: 11257478
-
Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy.Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8910-5. doi: 10.1073/pnas.1537554100. Epub 2003 Jul 8. Proc Natl Acad Sci U S A. 2003. PMID: 12851463 Free PMC article.
-
[The frequency of patients with adhalin deficiency in a muscular dystrophy patient population].Nihon Rinsho. 1997 Dec;55(12):3165-8. Nihon Rinsho. 1997. PMID: 9436429 Review. Japanese.
Cited by
-
Gene expression profiling of Duchenne muscular dystrophy skeletal muscle.Neurogenetics. 2003 Aug;4(4):163-71. doi: 10.1007/s10048-003-0148-x. Epub 2003 Apr 16. Neurogenetics. 2003. PMID: 12698323
-
Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice.Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1194-9. doi: 10.1073/pnas.98.3.1194. Epub 2001 Jan 23. Proc Natl Acad Sci U S A. 2001. PMID: 11158616 Free PMC article.
-
Secondary coronary artery vasospasm promotes cardiomyopathy progression.Am J Pathol. 2004 Mar;164(3):1063-71. doi: 10.1016/S0002-9440(10)63193-8. Am J Pathol. 2004. PMID: 14982859 Free PMC article.
-
Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy.Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13818-23. doi: 10.1073/pnas.250379497. Proc Natl Acad Sci U S A. 2000. PMID: 11087833 Free PMC article.
-
Recombinant annexin A6 promotes membrane repair and protects against muscle injury.J Clin Invest. 2019 Nov 1;129(11):4657-4670. doi: 10.1172/JCI128840. J Clin Invest. 2019. PMID: 31545299 Free PMC article.
References
-
- Campbell K P. Cell. 1995;80:675–679. - PubMed
-
- Bonnemann C G, McNally E M, Kunkel L M. Curr Opin Peds. 1996;8:569–582. - PubMed
-
- Straub V, Campbell K P. Curr Opin Neurol. 1997;10:168–175. - PubMed
-
- Lim L E, Campbell K P. Curr Opin Neurol. 1998;11:443–452. - PubMed
-
- Burghes A H, Logan C, Hu X, Belfall B, Worton R G, Ray P N. Nature (London) 1987;328:434–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

