Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux
- PMID: 10485908
- PMCID: PMC17965
- DOI: 10.1073/pnas.96.19.10812
Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux
Abstract
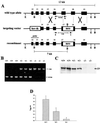
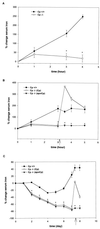
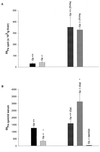
Aceruloplasminemia is an autosomal recessive disorder of iron metabolism. Affected individuals evidence iron accumulation in tissue parenchyma in association with absent serum ceruloplasmin. Genetic studies of such patients reveal inherited mutations in the ceruloplasmin gene. To elucidate the role of ceruloplasmin in iron homeostasis, we created an animal model of aceruloplasminemia by disrupting the murine ceruloplasmin (Cp) gene. Although normal at birth, Cp(-/-) mice demonstrate progressive accumulation of iron such that by one year of age all animals have a prominent elevation in serum ferritin and a 3- to 6-fold increase in the iron content of the liver and spleen. Histological analysis of affected tissues in these mice shows abundant iron stores within reticuloendothelial cells and hepatocytes. Ferrokinetic studies in Cp(+/+) and Cp(-/-) mice reveal equivalent rates of iron absorption and plasma iron turnover, suggesting that iron accumulation results from altered compartmentalization within the iron cycle. Consistent with this concept, Cp(-/-) mice showed no abnormalities in cellular iron uptake but a striking impairment in the movement of iron out of reticuloendothelial cells and hepatocytes. Our findings reveal an essential physiologic role for ceruloplasmin in determining the rate of iron efflux from cells with mobilizable iron stores.
Figures

References
-
- Ponka P, Beaumont C, Richardson D R. Semin Hematol. 1998;35:35–54. - PubMed
-
- Brittenham G M. In: Iron Metabolism in Health and Disease. Brock J H, Halliday J W, Pippard M J, Powell L W, editors. Philadelphia: Saunders; 1994. pp. 31–62.
-
- Andrews N C, Levy J E. Blood. 1998;92:1845–1851. - PubMed
-
- Bacon B R, Schilsky M L. Adv Intern Med. 1999;44:91–116. - PubMed
-
- Gitlin J D. Pediatr Res. 1998;44:271–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

