Orexin A activates locus coeruleus cell firing and increases arousal in the rat
- PMID: 10485925
- PMCID: PMC17982
- DOI: 10.1073/pnas.96.19.10911
Orexin A activates locus coeruleus cell firing and increases arousal in the rat
Abstract
The localization of orexin neuropeptides in the lateral hypothalamus has focused interest on their role in ingestion. The orexigenic neurones in the lateral hypothalamus, however, project widely in the brain, and thus the physiological role of orexins is likely to be complex. Here we describe an investigation of the action of orexin A in modulating the arousal state of rats by using a combination of tissue localization and electrophysiological and behavioral techniques. We show that the brain region receiving the densest innervation from orexinergic nerves is the locus coeruleus, a key modulator of attentional state, where application of orexin A increases cell firing of intrinsic noradrenergic neurones. Orexin A increases arousal and locomotor activity and modulates neuroendocrine function. The data suggest that orexin A plays an important role in orchestrating the sleep-wake cycle.
Figures

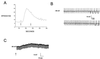
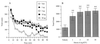
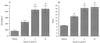
References
-
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemilli R M, Tanaka H, Clay Williams S, Richardson J A, Kozlowski G P, Wilson S, et al. Cell. 1998;92:573–585. - PubMed
-
- Elias C F, Saper C B, Maratos-Flier E, Tritos N A, Lee C, Kelly J, Tatro J B, Hoffman G E, Ollman M M, Barsh G S, et al. J Comp Neurol. 1998;402:442–459. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

