RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload
- PMID: 10487771
- PMCID: PMC408537
- DOI: 10.1172/JCI6713
RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload
Abstract
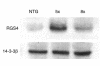
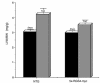
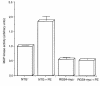
RGS family members are GTPase-activating proteins (GAPs) for heterotrimeric G proteins. There is evidence that altered RGS gene expression may contribute to the pathogenesis of cardiac hypertrophy and failure. We investigated the ability of RGS4 to modulate cardiac physiology using a transgenic mouse model. Overexpression of RGS4 in postnatal ventricular tissue did not affect cardiac morphology or basal cardiac function, but markedly compromised the ability of the heart to adapt to transverse aortic constriction (TAC). In contrast to wild-type mice, the transgenic animals developed significantly reduced ventricular hypertrophy in response to pressure overload and also did not exhibit induction of the cardiac "fetal" gene program. TAC of the transgenic mice caused a rapid decompensation in most animals characterized by left ventricular dilatation, depressed systolic function, and increased postoperative mortality when compared with nontransgenic littermates. These results implicate RGS proteins as a crucial component of the signaling pathway involved in both the cardiac response to acute ventricular pressure overload and the cardiac hypertrophic program.
Figures


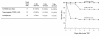



References
-
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. - PubMed
-
- Knowlton KU, et al. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during α-adrenergic stimulation of neonatal rat ventricular cells. J Biol Chem. 1991;266:7759–7768. - PubMed
-
- Lee H, et al. α1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells: effects on myosin light chain-2 gene expression. J Biol Chem. 1988;263:7352–7358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

