Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells
- PMID: 10487834
- PMCID: PMC1866901
- DOI: 10.1016/S0002-9440(10)65175-9
Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells
Abstract
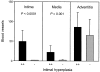
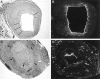
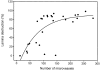
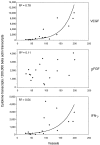
Inflammation of the arterial wall in giant cell arteritis induces a series of structural changes, including the formation of new vasa vasorum. To study the regulation of neoangiogenesis in giant cell arteritis, temporal arteries were examined for the extent and localization of microvessel generation and for the production of angiogenic factors. In normal arteries, vasa vasorum were restricted to the adventitia, but in inflamed arteries, capillaries emerged in the media and the intima. These capillaries displayed a distinct topography with a circumferential arrangement in the external one-third of the intima. Neovascularization was closely correlated with the formation of lumen-obstructing intima, the fragmentation of the internal elastic lamina, and the presence of multinucleated giant cells. Comparison of tissue cytokine transcription in temporal arteries of giant cell arteritis patients with and without up-regulated neoangiogenesis identified interferon-gamma and vascular endothelial growth factor but not fibroblast growth factor-2 as mediators associated with vasa vasorum proliferation. Giant cells and CD68-positive macrophages at the media-intima junction were found to be the major cellular sources of vascular endothelial growth factor. These data demonstrate that formation of new vasa vasorum in vasculitis is regulated by inflammatory cells and not by arterial wall cells, raising the possibility that it represents a primary disease mechanism and not a secondary hypoxia-induced event. Increased neovascularization in interferon-gamma-rich arteries suggests that the formation of new vasa vasorum is determined by the nature of the immune response in the arterial wall, possibly resulting from the generation and functional activity of multinucleated giant cells.
Figures


References
-
- Hunder GG: Giant cell arteritis and polymyalgia rheumatica. Med Clin North Am 1997, 81:195-219 - PubMed
-
- Nordborg E, Nordborg C, Malmvall BE, Andersson R, Bengtsson BA: Giant cell arteritis. Rheum Dis Clin North Am 1995, 21:1013-1026 - PubMed
-
- Kaiser M, Weyand CM, Bjornsson J, Goronzy JJ: Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum 1998, 41:623-633 - PubMed
-
- Evans JM, O’Fallon WM, Hunder GG: Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis: a population-based study. Ann Intern Med 1995, 122:502-507 - PubMed
-
- Weyand CM, Goronzy JJ: Giant cell arteritis as an antigen-driven disease. Rheum Dis Clin North Am 1995, 21:1027-1039 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

