Involvement of soluble CD95 in Churg-Strauss syndrome
- PMID: 10487849
- PMCID: PMC1866905
- DOI: 10.1016/S0002-9440(10)65191-7
Involvement of soluble CD95 in Churg-Strauss syndrome
Abstract
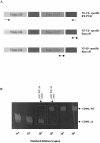
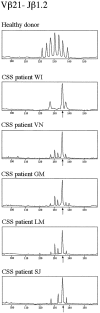
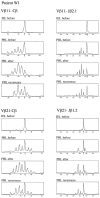
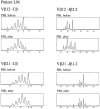
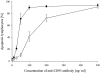
Deficiency of CD95 (Apo-1/Fas)-mediated apoptosis has recently been found in some autoimmune lymphoproliferative disorders due to inherited mutations of the CD95 gene. In this study, impairment of CD95 ligand-mediated killing of lymphocytes and eosinophils in Churg-Strauss Syndrome (CSS), which was a result of variation of CD95 receptor isoform expression, is demonstrated. Compared to those from healthy individuals, peripheral blood lymphocytes from eight CSS patients exhibit a switch from the membrane-bound CD95 receptor expression to its soluble splice variant, which protects from CD95L-mediated apoptosis. In five out of seven CSS patients recurrent oligoclonal T cell expansions were found, all using a Vbeta-gene from the Vbeta21 family associated with similar CDR3 motifs, indicating the predominance of T cell clones of a similar specificity in the CSS patients. In two of them, the effect of immunosuppressive therapy was studied. In both cases aberrant overexpression of the soluble CD95 receptor isoform and deviations from normal TCR Vbeta-gene usage normalized in parallel with the clinical improvement. Furthermore, soluble CD95 was identified as a survival factor for eosinophils rescuing eosinophils from apoptosis in the absence of growth factors in vitro. Given the role of eosinophils as effector cells in CSS, these findings suggest that soluble CD95 may be mechanistically involved in the disease.
Figures
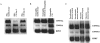

References
-
- Modigliani R, Muschart JM, Galian A, Clauvel JP, Desruisseaux JL: Allergic granulomatous vasculitis: report of a case with digestive involvement. Dig Dis Sci 1981, 26:264-270 - PubMed
-
- Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY: The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome. Arthritis Rheum 1990, 33:1094-1102 - PubMed
-
- Lanham GJ, Elkon KB, Pusey CD, Hughes GR: System vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine 1984, 63:65-81 - PubMed
-
- Schmitt WH, Csernok E, Kobayashi S, Klinkenborg A, Reinhold-Keller E, Gross WL: Churg-Strauss syndrome: serum markers of lymphocyte activation and endothelial damage. Arthritis Rheum 1998, 41:445-452 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

