Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36
- PMID: 10487979
- PMCID: PMC1866879
- DOI: 10.1016/s0002-9440(10)65183-8
Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36
Abstract
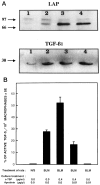
Transforming growth factor-beta-1 (TGF-beta1) is secreted by cells in a latent form (L-TGF-beta1) noncovalently bound to a latency-associated peptide. Activated alveolar macrophages obtained from rat lungs after bleomycin-induced pulmonary injury released increased amounts of active TGF-beta1 as well as plasmin, a protease, and thrombospondin-1 (TSP-1), a trimeric glycoprotein. Previously we had demonstrated that plasmin was critical to the activation of L-TGF- beta1. In the present study we demonstrated that TSP-1 is also important for the activation of L-TGF- beta1 because the activation can be inhibited by anti-TSP-1 monoclonal antibody. Proteins obtained from alveolar macrophage cell lysates immunoprecipitated with antibodies specific for TSP-1 were identified on immunoblots as LAP and TGF-beta1, indicating that TSP-1/L-TGF-beta1 complexes are present on alveolar macrophages. However, in the presence of plasmin both latency-associated peptide and TGF-beta1 were decreased in the same cell lysates, indicating that L-TGF-beta1 associated with TSP-1 is released by plasmin. Using immunofluorescence and antibodies to TGF-beta1 and CD36, a receptor for TSP-1, there was colocalization of TGF-beta1 with CD36. Because TSP-1 but not TGF-beta1 is a natural ligand for CD36, these findings suggest that the L-TGF-beta1 in a complex with TSP-1 localizes to the macrophage cell surface when TSP-1 interacts with its receptor, CD36. Furthermore, the association of TSP-1/L-TGF-beta1 complex with CD36 is necessary to the activation of L-TGF-beta1 because antibodies to CD36 prevent the colocalization of TGF-beta1 with CD36 as observed by immunofluorescence and inhibit activation of the L-TGF-beta1 by explanted alveolar macrophages. These findings suggest that activation of L-TGF-beta1 by plasmin occurs at the cell surface of activated alveolar macrophages and requires a TSP-1/CD36 interaction.
Figures





References
-
- Rappolee DA, Werb Z: Macrophage derived growth factors. Curr Topics Microbiol Immumol 1992, 181:87-140 - PubMed
-
- Stein M, Keshav A: The versatility of macrophages. Clin Exp Allergy 1992, 22:19-27 - PubMed
-
- Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera S, Rifkin DB: TGF-β latency: biological significance and mechanism of activation. Stem Cells 1997, 15:190-197 - PubMed
-
- Khalil N, Corne S, Whitman C, Yacyshyn H: Plasmin regulates the activation of cell associated latent TGF-β1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Resp Mol Cell Biol 1996, 15:252-259 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

