Protein kinase A regulates cholinergic gene expression in PC12 cells: REST4 silences the silencing activity of neuron-restrictive silencer factor/REST
- PMID: 10490617
- PMCID: PMC84675
- DOI: 10.1128/MCB.19.10.6788
Protein kinase A regulates cholinergic gene expression in PC12 cells: REST4 silences the silencing activity of neuron-restrictive silencer factor/REST
Abstract
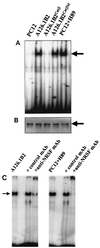
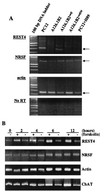
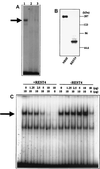
The role of protein kinase A in regulating transcription of the cholinergic gene locus, which contains both the vesicular acetylcholine transporter gene and the choline acetyltransferase gene, was investigated in PC12 cells and a protein kinase A-deficient PC12 mutant, A126.1B2, in which transcription of the gene is reduced. The site of action of protein kinase A was localized to a neuron-restrictive silencer element/repressor element 1 (NRSE/RE-1) sequence within the cholinergic gene. Neuron-restrictive silencer factor (NRSF)/RE-1-silencing transcription factor (REST), the transcription factor which binds to NRSE/RE-1, was expressed at similar levels in both PC12 and A126.1B2 cells. Although nuclear extracts containing NRSF/REST from A126.1B2 exhibited binding to NRSE/RE-1, nuclear extracts from PC12 cells did not. The NRSF/REST isoform REST4 was expressed in PC12 cells but not in A126.1B2. REST4 inhibited binding of NRSF/REST to NRSE/RE-1 as determined by gel mobility shift assays. Coimmunoprecipitation was used to demonstrate interaction between NRSF/REST and REST4. Expression of recombinant REST4 in A126.1B2 was sufficient to transcriptionally activate the cholinergic gene locus. Thus, in PC12 cells, protein kinase A promotes the production of REST4, which inhibits repression of the cholinergic gene locus by NRSF/REST.
Figures

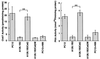


Similar articles
-
Regulation of cholinergic gene expression by the neuron restrictive silencer factor/repressor element-1 silencing transcription factor.Life Sci. 2003 Mar 28;72(18-19):2021-8. doi: 10.1016/s0024-3205(03)00065-1. Life Sci. 2003. PMID: 12628452
-
Regulation of the cholinergic gene locus by the repressor element-1 silencing transcription factor/neuron restrictive silencer factor (REST/NRSF).Life Sci. 2004 Mar 19;74(18):2213-25. doi: 10.1016/j.lfs.2003.08.045. Life Sci. 2004. PMID: 15017977 Review.
-
REST4-mediated modulation of REST/NRSF-silencing function during BDNF gene promoter activation.Biochem Biophys Res Commun. 2002 Jan 11;290(1):415-20. doi: 10.1006/bbrc.2001.6194. Biochem Biophys Res Commun. 2002. PMID: 11779185
-
Studies on the interaction of REST4 with the cholinergic repressor element-1/neuron restrictive silencer element.Brain Res Mol Brain Res. 2000 Aug 14;80(1):88-98. doi: 10.1016/s0169-328x(00)00129-7. Brain Res Mol Brain Res. 2000. PMID: 11039732
-
Brain REST/NRSF Is Not Only a Silent Repressor but Also an Active Protector.Mol Neurobiol. 2017 Jan;54(1):541-550. doi: 10.1007/s12035-015-9658-4. Epub 2016 Jan 7. Mol Neurobiol. 2017. PMID: 26742529 Review.
Cited by
-
Ischemic insults derepress the gene silencer REST in neurons destined to die.J Neurosci. 2003 Mar 15;23(6):2112-21. doi: 10.1523/JNEUROSCI.23-06-02112.2003. J Neurosci. 2003. PMID: 12657670 Free PMC article.
-
The transcriptional repressor REST determines the cell-specific expression of the human MAPK8IP1 gene encoding IB1 (JIP-1).Mol Cell Biol. 2001 Nov;21(21):7256-67. doi: 10.1128/MCB.21.21.7256-7267.2001. Mol Cell Biol. 2001. PMID: 11585908 Free PMC article.
-
An antisense amido-bridged nucleic acid gapmer oligonucleotide targeting SRRM4 alters REST splicing and exhibits anti-tumor effects in small cell lung cancer and prostate cancer cells.Cancer Cell Int. 2023 Jan 17;23(1):8. doi: 10.1186/s12935-022-02842-1. Cancer Cell Int. 2023. PMID: 36650528 Free PMC article.
-
The neuron-restrictive silencer element-neuron-restrictive silencer factor system regulates basal and endothelin 1-inducible atrial natriuretic peptide gene expression in ventricular myocytes.Mol Cell Biol. 2001 Mar;21(6):2085-97. doi: 10.1128/MCB.21.6.2085-2097.2001. Mol Cell Biol. 2001. PMID: 11238943 Free PMC article.
-
Expression patterns of mouse repressor element-1 silencing transcription factor 4 (REST4) and its possible function in neuroblastoma.J Mol Neurosci. 2000 Dec;15(3):205-14. doi: 10.1385/JMN:15:3:205. J Mol Neurosci. 2000. PMID: 11303784
References
-
- Bejanin S, Cervini R, Mallet J, Berrard S. A unique gene organization for two cholinergic markers, choline acetyltransferase and a putative vesicular transporter of acetylcholine. J Biol Chem. 1994;269:21944–21947. - PubMed
-
- Berrard S, Varoqui H, Cervini R, Israel M, Mallet J, Diebler M F. Coregulation of two embedded gene products, choline acetyltransferase and the vesicular acetylcholine transporter. J Neurochem. 1995;65:939–942. - PubMed
-
- Berse B, Blusztajn J K. Coordinated up-regulation of choline acetyltransferase and vesicular acetylcholine transporter gene expression by the retinoic acid receptor α, cAMP, and leukemia inhibitory factor/ciliary neurotrophic factor signaling pathways in a murine septal cell line. J Biol Chem. 1995;270:22101–22104. - PubMed
-
- Cassano S, Gallo A, Buccigrossi V, Porcellini A, Cerillo R, Gottesman M E, Avvedimento E V. Membrane localization of cAMP-dependent protein kinase amplifies cAMP signaling to the nucleus in PC12 cells. J Biol Chem. 1996;271:29870–29875. - PubMed
-
- Cervini R, Houhou L, Pradat P-F, Bejanin S, Mallet J, Berrard S. Specific vesicular acetylcholine transporter promoters lie within the first intron of the rat choline acetyltransferase gene. J Biol Chem. 1995;270:24654–24657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
