Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers
- PMID: 10490623
- PMCID: PMC84681
- DOI: 10.1128/MCB.19.10.6845
Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers
Abstract
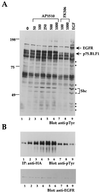
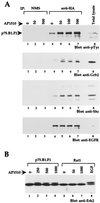
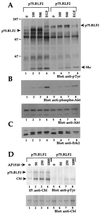
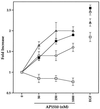
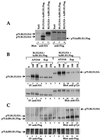
The four members of the ErbB family of receptor tyrosine kinases are involved in a complex array of combinatorial interactions involving homo- and heterodimers. Since most cell types express more than one member of the ErbB family, it is difficult to distinguish the biological activities of different homo- and heterodimers. Here we describe a method for inducing homo- or heterodimerization of ErbB receptors by using synthetic ligands without interference from the endogenous receptors. ErbB receptor chimeras containing synthetic ligand binding domains (FK506-binding protein [FKBP] or FKBP-rapamycin-binding domain [FRB]) were homodimerized with the bivalent FKBP ligand AP1510 and heterodimerized with the bifunctional FKBP-FRB ligand rapamycin. AP1510 treatment induced tyrosine phosphorylation of ErbB1 and ErbB2 homodimers and recruitment of Src homology 2 domain-containing proteins (Shc and Grb2). In addition, ErbB1 and ErbB2 homodimers activated downstream signaling pathways leading to Erk2 and Akt phosphorylation. However, only ErbB1 homodimers were internalized upon AP1510 stimulation, and only ErbB1 homodimers were able to associate with and induce phosphorylation of c-Cbl. Cells expressing AP1510-induced ErbB1 homodimers were able to associate with and induce phosphorylation of c-Cbl. Cells expressing AP1510-induced ErbB1 homodimers were able to form foci; however, cells expressing ErbB2 homodimers displayed a five- to sevenfold higher focus-forming ability. Using rapamycin-inducible heterodimerization we show that c-Cbl is unable to associate with ErbB1 in a ErbB1-ErbB2 heterodimer most likely because ErbB2 is unable to phosphorylate the c-Cbl binding site on ErbB1. Thus, we demonstrate that ErbB1 and ErbB2 homodimers differ in their abilities to transform fibroblasts and provide evidence for differential signaling by ErbB homodimers and heterodimers. These observations also validate the use of synthetic ligands to study the signaling and biological specificity of selected ErbB dimers in any cell type.
Figures




Similar articles
-
Controlled activation of ErbB1/ErbB2 heterodimers promote invasion of three-dimensional organized epithelia in an ErbB1-dependent manner: implications for progression of ErbB2-overexpressing tumors.Cancer Res. 2006 May 15;66(10):5201-8. doi: 10.1158/0008-5472.CAN-05-4081. Cancer Res. 2006. PMID: 16707444
-
The C-terminus of the kinase-defective neuregulin receptor ErbB-3 confers mitogenic superiority and dictates endocytic routing.EMBO J. 1999 Jun 15;18(12):3348-58. doi: 10.1093/emboj/18.12.3348. EMBO J. 1999. PMID: 10369675 Free PMC article.
-
Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins.J Biol Chem. 1996 Jun 14;271(24):14554-9. doi: 10.1074/jbc.271.24.14554. J Biol Chem. 1996. PMID: 8662998
-
The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions.FEBS Lett. 1997 Jun 23;410(1):83-6. doi: 10.1016/s0014-5793(97)00412-2. FEBS Lett. 1997. PMID: 9247128 Review.
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
Cited by
-
Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells.Proc Natl Acad Sci U S A. 2004 Feb 3;101(5):1257-62. doi: 10.1073/pnas.0308090100. Epub 2004 Jan 22. Proc Natl Acad Sci U S A. 2004. PMID: 14739340 Free PMC article.
-
Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells.Mol Cell Biol. 2004 Oct;24(19):8586-99. doi: 10.1128/MCB.24.19.8586-8599.2004. Mol Cell Biol. 2004. PMID: 15367678 Free PMC article.
-
Endocytic down-regulation of ErbB2 is stimulated by cleavage of its C-terminus.Mol Biol Cell. 2007 Sep;18(9):3656-66. doi: 10.1091/mbc.e07-01-0025. Epub 2007 Jul 11. Mol Biol Cell. 2007. PMID: 17626164 Free PMC article.
-
Movement of accessible plasma membrane cholesterol by the GRAMD1 lipid transfer protein complex.Elife. 2019 Nov 14;8:e51401. doi: 10.7554/eLife.51401. Elife. 2019. PMID: 31724953 Free PMC article.
-
A Generalizable Optogenetic Strategy to Regulate Receptor Tyrosine Kinases during Vertebrate Embryonic Development.J Mol Biol. 2020 May 1;432(10):3149-3158. doi: 10.1016/j.jmb.2020.03.032. Epub 2020 Apr 8. J Mol Biol. 2020. PMID: 32277988 Free PMC article.
References
-
- Alroy I, Yarden Y. The ErbB signaling network in embyrogensis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. - PubMed
-
- Baulida J, Kraus M H, Alimandi M, Di Fiore P P, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. - PubMed
-
- Belsches, A. P., M. D. Haskell, and S. J. Parsons. 1997. Role of c-Src tyrosine kinase in EGF-induced mitogenesis. Front. Biosci. 2:d501–d518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
