Inhibition of src family kinases by a combinatorial action of 5'-AMP and small heat shock proteins, identified from the adult heart
- PMID: 10490624
- PMCID: PMC84682
- DOI: 10.1128/MCB.19.10.6858
Inhibition of src family kinases by a combinatorial action of 5'-AMP and small heat shock proteins, identified from the adult heart
Abstract
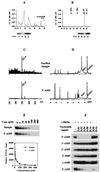
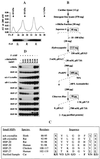
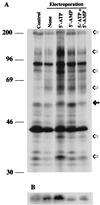
Src family kinases are implicated in cellular proliferation and transformation. Terminally differentiated myocytes have lost the ability to proliferate, indicating the existence of a down-regulatory mechanism(s) for these mitogenic kinases. Here we show that feline cardiomyocyte lysate contains thermostable components that inhibit c-Src kinase in vitro. This inhibitory activity, present predominantly in heart tissue, involves two components acting combinatorially. After purification by sequential chromatography, one component was identified by mass and nuclear magnetic resonance spectroscopies as 5'-AMP, while the other was identified by peptide sequencing as a small heat shock protein (sHSP). 5'-AMP and to a lesser extent 5'-ADP inhibit c-Src when combined with either HSP-27 or HSP-32. Other HSPs, including alphaB-crystallin, HSP-70, and HSP-90, did not exhibit this effect. The inhibition, observed preferentially on Src family kinases and independent of the Src tyrosine phosphorylation state, occurs via a direct interaction of the c-Src catalytic domain with the inhibitory components. Our study indicates that sHSPs increase the affinity of 5'-AMP for the c-Src ATP binding site, thereby facilitating the inhibition. In vivo, elevation of ATP levels in the cardiomyocytes results in the tyrosine phosphorylation of cellular proteins including c-Src at the activatory site, and this effect is blocked when the 5'-AMP concentration is raised. Thus, this study reveals a novel role for sHSPs and 5'-AMP in the regulation of Src family kinases, presumably for the maintenance of the terminally differentiated state.
Figures
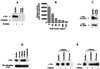


Similar articles
-
Purification of bovine thymus cytosolic C-terminal Src kinase (CSK) and demonstration of differential efficiencies of phosphorylation and inactivation of p56lyn and pp60c-src by CSK.Biochemistry. 1996 Sep 10;35(36):11874-87. doi: 10.1021/bi9603940. Biochemistry. 1996. PMID: 8794770
-
A dual inhibitor of platelet-derived growth factor beta-receptor and Src kinase activity potently interferes with motogenic and mitogenic responses to PDGF in vascular smooth muscle cells. A novel candidate for prevention of vascular remodeling.Circ Res. 1999 Jul 9;85(1):12-22. doi: 10.1161/01.res.85.1.12. Circ Res. 1999. PMID: 10400906
-
The pp60c-Src inhibitor PP1 is non-competitive against ATP.FEBS Lett. 2003 Feb 27;537(1-3):47-52. doi: 10.1016/s0014-5793(03)00069-3. FEBS Lett. 2003. PMID: 12606029
-
Endogenous and synthetic inhibitors of the Src-family protein tyrosine kinases.Biochim Biophys Acta. 2005 Dec 30;1754(1-2):210-20. doi: 10.1016/j.bbapap.2005.07.027. Epub 2005 Sep 8. Biochim Biophys Acta. 2005. PMID: 16198159 Review.
-
Structures of Src-family tyrosine kinases.Curr Opin Struct Biol. 1997 Dec;7(6):777-85. doi: 10.1016/s0959-440x(97)80146-7. Curr Opin Struct Biol. 1997. PMID: 9434895 Review.
References
-
- Arteaga C L, Ramsey T T, Shawver L K, Guyer C A. Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem. 1997;272:23247–23254. - PubMed
-
- Atanassov C L, Naegeli H U, Zenke G, Schneider C, Kramarova L I, Bronnikov G E, VanRegenmortel M H. Anti-lymphoproliferative activity of brown adipose tissue of hibernating ground squirrels is mainly caused by AMP. Comp Biochem Physiol C. 1995;112:93–100. - PubMed
-
- Benjamin I J, McMillan D R. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. - PubMed
-
- Boerner R J, Kassel D B, Edison A M, Knight W B. Examination of the dephosphorylation reactions catalyzed by pp60c-src tyrosine kinase explores the roles of autophosphorylation and SH2 ligand binding. Biochemistry. 1995;34:14852–14860. - PubMed
-
- Brown M T, Cooper J A. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
