Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice
- PMID: 10490643
- PMCID: PMC84701
- DOI: 10.1128/MCB.19.10.7061
Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice
Erratum in
-
Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice.Mol Cell Biol. 2006 Dec;26(24):9571. doi: 10.1128/MCB.01939-06. Mol Cell Biol. 2006. PMID: 17135280 Free PMC article. No abstract available.
Abstract
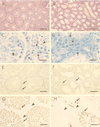
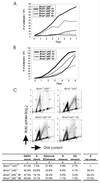
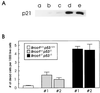
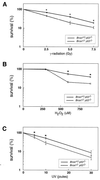
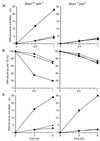
BRCA1 is a nuclear phosphoprotein expressed in a broad spectrum of tissues during cell division. The inheritance of a mutant BRCA1 allele dramatically increases a woman's lifetime risk for developing both breast and ovarian cancers. A number of mouse lines carrying mutations in the Brca1 gene have been generated, and mice homozygous for these mutations generally die before day 10 of embryonic development. We report here the survival of a small number of mice homozygous for mutations in both the p53 and Brca1 genes. The survival of these mice is likely due to additional unknown mutations or epigenetic effects. Analysis of the Brca1(-/-) p53(-/-) animals indicates that BRCA1 is not required for the development of most organ systems. However, these mice are growth retarded, males are infertile due to meiotic failure, and the mammary gland of the female mouse is underdeveloped. Growth deficiency due to loss of BRCA1 was more thoroughly examined in an analysis of primary fibroblast lines obtained from these animals. Like p53(-/-) fibroblasts, Brca1(-/-) p53(-/-) cells proliferate more rapidly than wild-type cells; however, a high level of cellular death in these cultures results in reduced overall growth rates in comparison to p53(-/-) fibroblasts. Brca1(-/-) p53(-/-) fibroblasts are also defective in transcription-coupled repair and display increased sensitivity to DNA-damaging agents. We show, however, that after continued culture, and perhaps accelerated by the loss of BRCA1 repair functions, populations of Brca1(-/-) p53(-/-) fibroblasts with increased growth rates can be isolated. The increased survival of BRCA1-deficient fibroblasts in the absence of p53, and with the subsequent accumulation of additional growth-promoting changes, may mimic the events that occur during malignant transformation of BRCA1-deficient epithelia.
Figures



Comment in
-
Findings of scientific misconduct.NIH Guide Grants Contracts (Bethesda). 2006 Jun 9:NOT-OD-06-075. NIH Guide Grants Contracts (Bethesda). 2006. PMID: 16764107 Free PMC article. No abstract available.
-
Findings of Scientific Misconduct.Fed Regist. 2006 Jun 8;71(110):33308-33309. Fed Regist. 2006. PMID: 27737147 Free PMC article. No abstract available.
References
-
- Akashi M, Hachiya M, Osawa W, Spirin K, Suzuki G, Koeffler H. Irradiation induces WAF1 expression through a p53-independent pathway in KG-1 cells. J Biol Chem. 1995;270:19181–19187. - PubMed
-
- Blackshear P E, Goldsworthy S M, Foley J F, McAllister K A, Bennett L M, Collins N K, Bunch D O, Brown P, Wiseman R, Davis B J. Brca1 and Brca2 expression patterns in mitotic and meiotic cells of mice. Oncogene. 1998;16:61–68. - PubMed
-
- Callebaut I, Mornon J-P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. - PubMed
-
- Chapman M S, Verma I M. Transcriptional activation by BRCA1. Nature. 1996;382:678–679. - PubMed
-
- Chen C-F, Li S, Chen Y, Chen P-L, Sharp Z D, Lee W-H. The nuclear localization sequences of the BRCA1 protein interact with the importin-α subunit of the nuclear transport signal receptor. Proc Natl Acad Sci USA. 1996;271:32863–32868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
