Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis
- PMID: 10490655
- PMCID: PMC84713
- DOI: 10.1128/MCB.19.10.7203
Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis
Abstract
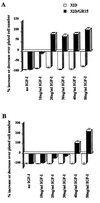
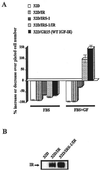
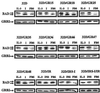
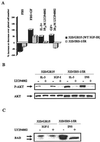
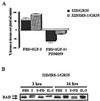
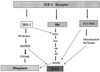
The type 1 insulin-like growth factor receptor (IGF-1R), activated by its ligands, protects several cell types from a variety of apoptotic injuries. The main signaling pathway for IGF-1R-mediated protection from apoptosis has been previously elucidated and rests on the activation of phosphatidylinositol 3-kinase, Akt/protein kinase B, and the phosphorylation and inactivation of BAD, a member of the Bcl-2 family of proteins. In 32D cells (a murine hemopoietic cell line devoid of insulin receptor substrate 1 [IRS-1]), the IGF-1R activates alternative pathways for protection from apoptosis induced by withdrawal of interleukin-3. One of these pathways leads to the activation of mitogen-activated protein kinase, while a third pathway results in the mitochondrial translocation of Raf and depends on the integrity of a group of serines in the C terminus of the receptor that are known to interact with 14.3.3 proteins. All three pathways, however, result in BAD phosphorylation. The presence of multiple antiapoptotic pathways may explain the remarkable efficacy of the IGF-1R in protecting cells from apoptosis.
Figures





References
-
- Avruch J. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem. 1998;182:31–48. - PubMed
-
- Backer J M, Myers M G, Jr, Sun X J, Chi D J, Shoelson S E, Miralpeix M, White M F. Association of IRS-1 with insulin receptor and the phosphatidylinositol 3′-kinase. J Biol Chem. 1993;268:8204–8212. - PubMed
-
- Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:105–126. - PubMed
-
- Baserga R. The price of independence. Exp Cell Res. 1997;236:1–3. - PubMed
-
- Brunet A, Bonni A, Zigmond M J, Lin M Z, Jun P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
