Op18/stathmin mediates multiple region-specific tubulin and microtubule-regulating activities
- PMID: 10491392
- PMCID: PMC2156119
- DOI: 10.1083/jcb.146.6.1289
Op18/stathmin mediates multiple region-specific tubulin and microtubule-regulating activities
Abstract
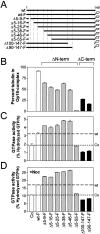
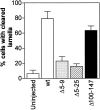
Oncoprotein18/stathmin (Op18) is a regulator of microtubule (MT) dynamics that binds tubulin heterodimers and destabilizes MTs by promoting catastrophes (i.e., transitions from growing to shrinking MTs). Here, we have performed a deletion analysis to mechanistically dissect Op18 with respect to (a) modulation of tubulin GTP hydrolysis and exchange, (b) tubulin binding in vitro, and (c) tubulin association and MT-regulating activities in intact cells. The data reveal distinct types of region-specific Op18 modulation of tubulin GTP metabolism, namely inhibition of nucleotide exchange and stimulation or inhibition of GTP hydrolysis. These regulatory activities are mediated via two-site cooperative binding to tubulin by multiple nonessential physically separated regions of Op18. In vitro analysis revealed that NH(2)- and COOH-terminal truncations of Op18 have opposite effects on the rates of tubulin GTP hydrolysis. Transfection of human leukemia cells with these two types of mutants result in similar decrease of MT content, which in both cases appeared independent of a simple tubulin sequestering mechanism. However, the NH(2)- and COOH-terminal-truncated Op18 mutants regulate MTs by distinct mechanisms as evidenced by morphological analysis of microinjected newt lung cells. Hence, mutant analysis shows that Op18 has the potential to regulate tubulin/MTs by more than one specific mechanism.
Figures






Similar articles
-
Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin.Mol Biol Cell. 1999 Jan;10(1):105-18. doi: 10.1091/mbc.10.1.105. Mol Biol Cell. 1999. PMID: 9880330 Free PMC article.
-
Mutational analysis of op18/stathmin-tubulin-interacting surfaces. Binding cooperativity controls tubulin GTP hydrolysis in the ternary complex.J Biol Chem. 2000 Nov 17;275(46):35759-66. doi: 10.1074/jbc.M005875200. J Biol Chem. 2000. PMID: 10954719
-
Molecular dissection of GTP exchange and hydrolysis within the ternary complex of tubulin heterodimers and Op18/stathmin family members.J Biol Chem. 2003 May 9;278(19):16651-7. doi: 10.1074/jbc.M300131200. Epub 2003 Feb 26. J Biol Chem. 2003. PMID: 12606544
-
The oncoprotein 18/stathmin family of microtubule destabilizers.Curr Opin Cell Biol. 2002 Feb;14(1):18-24. doi: 10.1016/s0955-0674(01)00289-7. Curr Opin Cell Biol. 2002. PMID: 11792540 Review.
-
Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin.Cell Struct Funct. 1999 Oct;24(5):345-57. doi: 10.1247/csf.24.345. Cell Struct Funct. 1999. PMID: 15216892 Review.
Cited by
-
Phosphorylation disrupts the central helix in Op18/stathmin and suppresses binding to tubulin.EMBO Rep. 2001 Jun;2(6):505-10. doi: 10.1093/embo-reports/kve105. EMBO Rep. 2001. PMID: 11415983 Free PMC article.
-
The Microtubule Regulatory Protein Stathmin Is Required to Maintain the Integrity of Axonal Microtubules in Drosophila.PLoS One. 2013 Jun 26;8(6):e68324. doi: 10.1371/journal.pone.0068324. Print 2013. PLoS One. 2013. PMID: 23840848 Free PMC article.
-
Molecular signatures of noncancerous liver tissue can predict the risk for late recurrence of hepatocellular carcinoma.J Gastroenterol. 2010 Feb;45(2):146-52. doi: 10.1007/s00535-009-0164-1. Epub 2009 Dec 8. J Gastroenterol. 2010. PMID: 19997856 Review.
-
Identification of a novel microtubule-destabilizing motif in CPAP that binds to tubulin heterodimers and inhibits microtubule assembly.Mol Biol Cell. 2004 Jun;15(6):2697-706. doi: 10.1091/mbc.e04-02-0121. Epub 2004 Mar 26. Mol Biol Cell. 2004. PMID: 15047868 Free PMC article.
-
The catastrophe-promoting activity of ectopic Op18/stathmin is required for disruption of mitotic spindles but not interphase microtubules.Mol Biol Cell. 2001 Jan;12(1):73-83. doi: 10.1091/mbc.12.1.73. Mol Biol Cell. 2001. PMID: 11160824 Free PMC article.
References
-
- Belmont L.D, Mitchison T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed
-
- Brattsand G., Roos G., Marklund U., Ueda H., Landberg G., Nanberg E., Sideras P., Gullberg M. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein 18) in normal and neoplastic cells. Leukemia. 1993;7:569–579. - PubMed
-
- Brylawski B.P., Caplow M. Rate for nucleotide release from tubulin. J. Biol. Chem. 1983;258:760–763. - PubMed
-
- Caplow M., Shanks J. Induction of microtubule catastrophe by formation of tubulin-GDP and apotubulin subunits at microtubule ends. Biochemistry. 1995;34:15732–15741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

