Growth factor modulation of substrate-specific morphological patterns in Aplysia bag cell neurons
- PMID: 10492011
- PMCID: PMC311303
Growth factor modulation of substrate-specific morphological patterns in Aplysia bag cell neurons
Abstract
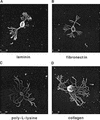
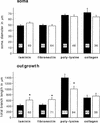
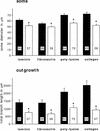
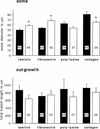
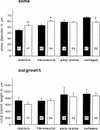
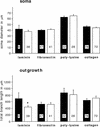
Components of the extracellular matrix (ECM) can act not only as passive substrates for neuronal attachment and outgrowth but also as active sites for signal transduction. Thus, specific ECM components may modulate effects of growth factors (GFs) that play an important role in structural changes in development and adult neuronal plasticity. In this study we examined the interaction of cultured Aplysia bag cell neurons (BCNs) with components of ECM and different GFs. Different ECM substrata induce a substrate-specific BCN morphology: BCNs grown on collagen or poly-L-lysine have larger soma diameter and more extensive neurite outgrowth than BCNs grown on laminin or fibronectin. BCNs also interact in a substrate-dependent way with GFs: BDNF treatment leads to a reduction of outgrowth on poly-L-lysine but an enhancement on fibronectin and laminin. CNTF reduces the soma diameter on collagen IV but enlarges it on laminin or fibronectin. In contrast, NGF induces a reduction of both soma diameter and outgrowth, on all substrata. Plating of BCNs in the presence of anti-beta1-integrin reduces adhesion to fibronectin but does not change outgrowth. In contrast, RGD peptides block adhesion to laminin and poly-L-lysine and, additionally, reduce outgrowth on laminin. These data suggest that BCNs use different beta1-integrin-dependent as well as RGD-dependent mechanisms for adhesion and outgrowth on different ECM substrata, providing possible sites of modulation by specific GFs.
Figures

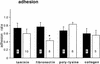
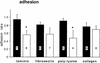
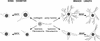
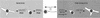
Similar articles
-
Endogenous neurotrophic factors enhance neurite growth by bag cell neurons of Aplysia.J Neurobiol. 2003 Jul;56(1):78-93. doi: 10.1002/neu.10221. J Neurobiol. 2003. PMID: 12767034
-
Neurite outgrowth, RGD-dependent, and RGD-independent adhesion of identified molluscan motoneurons on selected substrates.J Neurobiol. 1998 Apr;35(1):37-52. J Neurobiol. 1998. PMID: 9552165
-
Manganese induces spreading and process outgrowth in rat pheochromocytoma (PC12) cells.J Neurosci Res. 1993 Apr 1;34(5):546-61. doi: 10.1002/jnr.490340507. J Neurosci Res. 1993. PMID: 8386776
-
In vitro studies on the control of nerve fiber growth by the extracellular matrix of the nervous system.J Physiol (Paris). 1987;82(4):258-70. J Physiol (Paris). 1987. PMID: 3332689 Review.
-
Nerve growth factors and molecules of the extracellular matrix in neuronal development.J Cell Sci Suppl. 1985;3:107-13. doi: 10.1242/jcs.1985.supplement_3.11. J Cell Sci Suppl. 1985. PMID: 3914988 Review.
Cited by
-
A novel postsynaptic mechanism for heterosynaptic sharing of short-term plasticity.J Neurosci. 2010 Jun 30;30(26):8797-806. doi: 10.1523/JNEUROSCI.4767-09.2010. J Neurosci. 2010. PMID: 20592201 Free PMC article.
-
Pattern detection in the TGFβ cascade controls the induction of long-term synaptic plasticity.Proc Natl Acad Sci U S A. 2023 Oct 3;120(40):e2300595120. doi: 10.1073/pnas.2300595120. Epub 2023 Sep 25. Proc Natl Acad Sci U S A. 2023. PMID: 37748056 Free PMC article.
-
Calcineurin-dependent cofilin activation and increased retrograde actin flow drive 5-HT-dependent neurite outgrowth in Aplysia bag cell neurons.Mol Biol Cell. 2012 Dec;23(24):4833-48. doi: 10.1091/mbc.E12-10-0715. Epub 2012 Oct 24. Mol Biol Cell. 2012. PMID: 23097492 Free PMC article.
-
Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain.PLoS One. 2007 Jun 27;2(6):e573. doi: 10.1371/journal.pone.0000573. PLoS One. 2007. PMID: 17593972 Free PMC article.
-
Ca2+ removal by the plasma membrane Ca2+-ATPase influences the contribution of mitochondria to activity-dependent Ca2+ dynamics in Aplysia neuroendocrine cells.J Neurophysiol. 2016 Jun 1;115(5):2615-34. doi: 10.1152/jn.00494.2015. Epub 2016 Feb 10. J Neurophysiol. 2016. PMID: 26864756 Free PMC article.
References
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. - PubMed
-
- Barde Y-A. The nerve growth factor family. Prog Growth Factor Res. 1990;2:237–248. - PubMed
-
- Bogaert T, Brown N, Wilcox M. The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987;51:929–940. - PubMed
-
- Bulloch AGM, Syed NI, Ridgway RL. Neurite outgrowth and synapse formation by Lymnaea neurons: Towards a characterization of molluscan neurotrophic factors. Netherlands J Zool. 1994;44(3–4):317–326.
-
- Bunch TA, Brower DL. Drosophila PS2 integrin mediates RGD-dependent cell-matrix interactions. Development. 1992;116:239–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
