Inflammation causes a long-term hyperexcitability in the nociceptive sensory neurons of Aplysia
- PMID: 10492014
- PMCID: PMC311296
Inflammation causes a long-term hyperexcitability in the nociceptive sensory neurons of Aplysia
Abstract
Nerve injury, tissue damage, and inflammation all cause hyperalgesia. A factor contributing to this increased sensitivity is a long-term (>24 hr) hyperexcitability (LTH) in the sensory neurons that mediate the responses. Using the cluster of nociceptive sensory neurons in Aplysia californica as a model, we are examining how inflammation induces LTH. A general inflammatory response was induced by inserting a gauze pad into the animal Within 4 days, the gauze is enmeshed in an amorphous material that contains hemocytes, which comprise a cellular immune system. Concurrently, LTH appears in both ipsilateral and contralateral sensory neurons. The LTH is manifest as increased action potential discharge to a normalized stimulus. Immunocytochemistry revealed that hemocytes have antigens recognized by antibodies to TGFbeta1, IL-6, and 5HT. When a localized inflammation was elicited on a nerve, hemocytes containing the TGFbeta1 antigen were present near axons within the nerve and those containing the IL-6 were on the surface. Western blots of hemocytes, or of gauze that had induced a foreign body response, contained a 28-kD polypeptide recognized by the anti-TGFbeta1 antibody. Exposure of the nervous system to recombinant human TGFbeta1 elicited increased firing of the nociceptive neurons and a decrease in threshold. The TGFbeta1 also caused an activation of protein kinase C (PKC) in axons but did not affect a kinase that is activated in axons after injury. Our findings, in conjunction with previous results, indicate that a TGFbeta1-homolog can modulate the activity of neurons that respond to noxious stimuli. This system could also contribute to interactions between the immune and nervous systems via regulation of PKC.
Figures

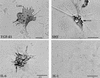
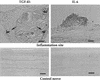

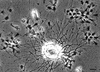
Similar articles
-
Serotonin induces memory-like, rapamycin-sensitive hyperexcitability in sensory axons of aplysia that contributes to injury responses.J Neurophysiol. 2007 Sep;98(3):1231-9. doi: 10.1152/jn.01189.2006. Epub 2007 Jul 18. J Neurophysiol. 2007. PMID: 17634332
-
Activation and retrograde transport of protein kinase G in rat nociceptive neurons after nerve injury and inflammation.Neuroscience. 2006 Aug 25;141(2):697-709. doi: 10.1016/j.neuroscience.2006.04.033. Epub 2006 May 30. Neuroscience. 2006. PMID: 16730916
-
Immune-mediated alterations in nociceptive sensory function in Aplysia californica.J Exp Biol. 1999 Mar;202(Pt 5):623-30. doi: 10.1242/jeb.202.5.623. J Exp Biol. 1999. PMID: 9929463
-
Pathways that elicit long-term changes in gene expression in nociceptive neurons following nerve injury: contributions to neuropathic pain.Neurol Res. 2004 Mar;26(2):195-203. doi: 10.1179/016164104225013761. Neurol Res. 2004. PMID: 15072639 Review.
-
Neural-immune interactions--an evolutionary perspective.Neuroimmunomodulation. 1998 May-Aug;5(3-4):136-42. doi: 10.1159/000026330. Neuroimmunomodulation. 1998. PMID: 9730679 Review.
Cited by
-
Nociceptive Biology of Molluscs and Arthropods: Evolutionary Clues About Functions and Mechanisms Potentially Related to Pain.Front Physiol. 2018 Aug 3;9:1049. doi: 10.3389/fphys.2018.01049. eCollection 2018. Front Physiol. 2018. PMID: 30123137 Free PMC article. Review.
-
In search of the Aplysia immunome: an in silico study.BMC Genomics. 2022 Jul 29;23(1):543. doi: 10.1186/s12864-022-08780-6. BMC Genomics. 2022. PMID: 35906538 Free PMC article.
-
Comparative biology of pain: What invertebrates can tell us about how nociception works.J Neurophysiol. 2017 Apr 1;117(4):1461-1473. doi: 10.1152/jn.00600.2016. Epub 2017 Jan 4. J Neurophysiol. 2017. PMID: 28053241 Free PMC article. Review.
-
Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory.Neurobiol Learn Mem. 2013 Oct;105:133-50. doi: 10.1016/j.nlm.2013.06.008. Epub 2013 Jun 22. Neurobiol Learn Mem. 2013. PMID: 23796633 Free PMC article. Review.
-
A neuronal isoform of protein kinase G couples mitogen-activated protein kinase nuclear import to axotomy-induced long-term hyperexcitability in Aplysia sensory neurons.J Neurosci. 2004 Aug 25;24(34):7583-95. doi: 10.1523/JNEUROSCI.1445-04.2004. J Neurosci. 2004. PMID: 15329406 Free PMC article.
References
-
- Ambron RT, Walters ET. Priming events and retrograde injury signals: A new perspective on the cellular and molecular biology of nerve regeneration. Mol Neurobiol. 1996;13:61–79. - PubMed
-
- Bayne CJ. Molluscan immunobiology. In: Saleuddin ASM, Wilbur KM, editors. The mollusca, Vol. 5, Physiology 2. New York, NY: Academic Press; 1983. pp. 407–486.
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in mammals. Pain. 1988;33:87–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
