Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons
- PMID: 10493721
- PMCID: PMC6783007
- DOI: 10.1523/JNEUROSCI.19-19-08199.1999
Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons
Abstract
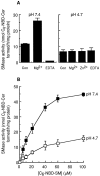
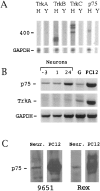
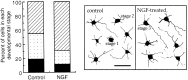
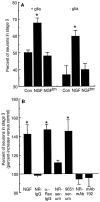
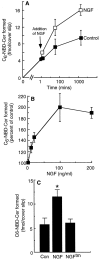
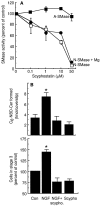
The p75 neurotrophin receptor (p75NTR) binds all known neurotrophins and has been suggested to either function as a coreceptor for the trk receptor tyrosine kinases or be involved in independent signaling leading to cell death. We have analyzed the effects of nerve growth factor (NGF) on the growth of cultured hippocampal pyramidal neurons and examined the possibility that the effects of NGF are mediated via generation of ceramide produced by neutral sphingomyelinase (N-SMase). During the initial hour of culture, the only detectable NGF receptor is p75NTR, which by comparative Western blot is expressed at 50- to 100-fold lower levels than on PC12 cells. At this early stage of culture, NGF accelerates neurite formation and outgrowth and induces ceramide formation in a dose-dependent manner. An NGF mutant that is deficient in p75NTR binding has no effect on neuronal morphology or ceramide formation. Furthermore, two anti-p75NTR antibodies (REX and 9651), which are known to compete with NGF for binding to p75NTR, mimic the effects of NGF, whereas a monoclonal antibody (MC192) targeted against a different epitope does not. Finally, scyphostatin, a specific N-SMase inhibitor, blocks the effects of NGF. We propose that a neurotrophin-p75NTR-ceramide signaling pathway influences outgrowth of hippocampal neurons. This signaling role of p75NTR may be distinct from other signaling pathways that lead to apoptosis.
Figures
References
-
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Morgener J, Kronke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. - PubMed
-
- Akar CA, Wallace WC. Amyloid precursor protein modulates the interaction of nerve growth factor with p75 receptor and potentiates its activation of trkA phosphorylation. Brain Res Mol Brain Res. 1998;56:125–132. - PubMed
-
- Barbacid M. Structural and functional properties of the Trk family of neurotrophin receptors. Ann NY Acad Sci. 1995;766:442–558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
