delta opioid receptor modulation of several voltage-dependent Ca(2+) currents in rat sensory neurons
- PMID: 10493735
- PMCID: PMC6783030
- DOI: 10.1523/JNEUROSCI.19-19-08337.1999
delta opioid receptor modulation of several voltage-dependent Ca(2+) currents in rat sensory neurons
Abstract
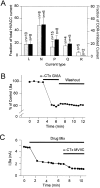
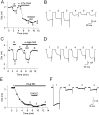
Endogenous enkephalins and delta opiates affect sensory function and pain sensation by inhibiting synaptic transmission in sensory circuits via delta opioid receptors (DORs). DORs have long been suspected of mediating these effects by modulating voltage-dependent Ca(2+) entry in primary sensory neurons. However, not only has this hypothesis never been validated in these cells, but in fact several previous studies have only turned up negative results. By using whole-cell current recordings, we show that the delta enkephalin analog [D-Ala(2), D-Leu(5)]-enkephalin (DADLE) inhibits, via DORs, L-, N-, P-, and Q-high voltage-activated Ca(2+) channel currents in cultured rat dorsal root ganglion (DRG) neurons. The percentage of responding cells was remarkably high (75%) within a novel subpopulation of substance P-containing neurons compared with the other cells (18-35%). DADLE (1 microM) inhibited 32% of the total barium current through calcium channels (I(Ba)). A delta (naltrindole, 1 microM), but not a mu (beta-funaltrexamine, 5 microM), antagonist prevented the DADLE response, whereas a DOR-2 subtype (deltorphin-II, 100 nM), but not a DOR-1 (DPDPE, 1 microM), agonist mimicked the response. L-, N-, P-, and Q-type currents contributed, on average, 18, 48, 14, and 16% to the total I(Ba) and 19, 50, 26, and 20% to the DADLE-sensitive current, respectively. The drug-insensitive R-type current component was not affected by the agonist. This work represents the first demonstration that DORs modulate Ca(2+) entry in sensory neurons and suggests that delta opioids could affect diverse Ca(2+)-dependent processes linked to Ca(2+) influx through different high-voltage-activated channel types.
Figures




References
-
- Albillos A, Carbone E, Gandia L, Garcia A, Pollo A. Opioid inhibition of Ca2+ channel subtypes in bovine chromaffin cells: selectivity of action and voltage-dependence. Eur J Neurosci. 1996;8:1561–1570. - PubMed
-
- Al Zein M, Lutz-Bucher B, Koch B. Modulation by leu-enkephalin of peptide release from perifused neurointermediate pituitary. I. Selective effect on potassium-, veratridine- and isoproterenol-stimulated secretion of vasopressin. Neuroendocrinology. 1984;39:392–396. - PubMed
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
