Identification of a new exon in the myelin proteolipid protein gene encoding novel protein isoforms that are restricted to the somata of oligodendrocytes and neurons
- PMID: 10493736
- PMCID: PMC6783048
- DOI: 10.1523/JNEUROSCI.19-19-08349.1999
Identification of a new exon in the myelin proteolipid protein gene encoding novel protein isoforms that are restricted to the somata of oligodendrocytes and neurons
Abstract
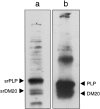
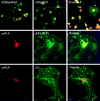
The myelin proteolipid protein (PLP) gene (i.e., the PLP/DM20 gene) has been of some interest because of its role in certain human demyelinating diseases, such as Pelizaeus-Merzbacher disease. A substantial amount of evidence, including neuronal pathology in knock-out and transgenic animals, suggests the gene also has functions unrelated to myelin structure, but the products of the gene responsible for these putative functions have not yet been identified. Here we report the identification of a new exon of the PLP/DM20 gene and at least two new products of the gene that contain this exon. The new exon, located between exons 1 and 2, is spliced into PLP and DM20 mRNAs creating a new translation initiation site that generates PLP and DM20 proteins with a 12 amino acid leader sequence. This leader sequence appears to target these proteins to a different cellular compartment within the cell bodies of oligodendrocytes and away from the myelin membranes. Furthermore, these new products are also expressed in a number of neuronal populations within the postnatal mouse brain, including the cerebellum, hippocampus, and olfactory system. We term these products somal-restricted PLP and DM20 proteins to distinguish them from the classic PLP and DM20 proteolipids. They represent putative candidates for some of the nonmyelin-related functions of the PLP/DM20 gene.
Figures




References
-
- Anderson TJ, Schneider A, Barrie JA, Klugmann M, McCulloch MC, Kirkham D, Kyriakides E, Nave KA, Griffiths IR. Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. J Comp Neurol. 1998;394:506–519. - PubMed
-
- Bizzozero OA, Besio-Moreno M, Pasquini JM, Soto EF, Gomez CJ. An electrophoretic analysis of proteolipids from different rat brain subcellular fractions. Biochim Biophys Acta. 1982;691:281–292. - PubMed
-
- Bongarzone ER, Foster LM, Byravan S, Verity AN, Landry CF, Schonmann V, Amur-Umarjee S, Campagnoni AT. Conditionally immortalized neural cell lines: potential models for the study of neural cell function. Methods. 1996;10:489–500. - PubMed
-
- Bongarzone ER, Foster L, Byravan S, Casaccia-Bonnefil P, Schonmann V, Campagnoni AT. Two neuronal cell lines expressing the Golli-MBP gene display differences in their in vitro survival and in their interaction with glia. J Neurosci Res. 1998b;54:309–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
