Drosophila presenilin is required for neuronal differentiation and affects notch subcellular localization and signaling
- PMID: 10493744
- PMCID: PMC6783002
- DOI: 10.1523/JNEUROSCI.19-19-08435.1999
Drosophila presenilin is required for neuronal differentiation and affects notch subcellular localization and signaling
Abstract
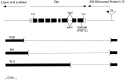
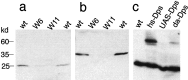
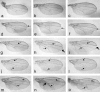
Presenilins are a highly conserved family of proteins first identified as causative genes in early onset familial Alzheimer's disease. Recent studies have suggested a role for presenilins in the Notch-signaling pathway, but their specific function within this pathway remains unclear. Here, we have characterized the Drosophila presenilin gene and protein and studied their interaction with Notch in both mutants and transgenics. We find that the Drosophila presenilin protein is proteolytically cleaved and broadly expressed during development with the highest levels in neurons within the larval CNS. We also show that mutations in Drosophila presenilin (Dps) genetically interact with Notch and result in an early pupal-lethal phenotype characterized by defects in eye and wing development and incomplete neuronal differentiation within the larval CNS. Moreover, we find that processing of Notch in the Golgi by the furin protease is unaffected in Dps mutants and that Notch is present and may even accumulate on the plasma membrane of neuroblasts in the larval CNS of Dps mutants. In contrast, overexpression of Dps in transgenics causes Notch to accumulate in the cytoplasm. Taken together, these results indicate that Drosophila presenilin is required for proper neuronal differentiation and may regulate the subcellular localization of Notch proteins within cells, necessary for their accumulation and subsequent signaling capabilities.
Figures




References
-
- Baumeister R, Leimer U, Zweckbronner I, Jakubek C, Grunberg J, Haass C. Human presenilin-1, but not familial Alzheimer’s disease (FAD) mutants, facilitate Caenorhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct. 1997;1:149–159. - PubMed
-
- Berezovska O, Xia M, Page K, Wasco W, Tanzi R, Hyman B. Developmental regulation of presenilin mRNA expression parallels Notch expression. J Neuropathol Exp Neurol. 1997;56:40–44. - PubMed
-
- Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. - PubMed
-
- Blochlinger K, Jan LY, Jan YN. Postembryonic patterns of expression of cut, a locus regulating sensory organ identity in Drosophila. Development. 1993;117:441–450. - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada C-M, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
