Ca(2+)-permeable AMPA receptors and spontaneous presynaptic transmitter release at developing excitatory spinal synapses
- PMID: 10493753
- PMCID: PMC6783006
- DOI: 10.1523/JNEUROSCI.19-19-08528.1999
Ca(2+)-permeable AMPA receptors and spontaneous presynaptic transmitter release at developing excitatory spinal synapses
Abstract
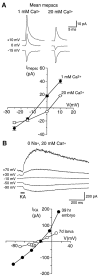
At many mature vertebrate glutamatergic synapses, excitatory transmission strength and plasticity are regulated by AMPA and NMDA receptor (AMPA-R and NMDA-R) activation and by patterns of presynaptic transmitter release. Both receptors potentially direct neuronal differentiation by mediating postsynaptic Ca(2+) influx during early development. However, the development of synaptic receptor expression and colocalization has been examined developmentally in only a few systems, and changes in release properties at neuronal synapses have not been characterized extensively. We recorded miniature EPSCs (mEPSCs) from spinal interneurons in Xenopus embryos and larvae. In mature 5-8 d larvae, approximately 70% of mEPSCs in Mg(2+)-free saline are composed of both a fast AMPA-R-mediated component and a slower NMDA-R-mediated decay, indicating receptor colocalization at most synapses. By contrast, in 39-40 hr embryos approximately 65% of mEPSCs are exclusively fast, suggesting that these synapses initially express predominantly AMPA-R. In a physiological Mg(2+) concentration (1 mM), mEPSCs throughout development are mainly AMPA-R-mediated at negative potentials. Embryonic synaptic AMPA-R are highly Ca(2+)-permeable, mEPSC amplitude is over twofold larger than at mature synapses, and mEPSCs frequently occur in bursts consistent with asynchronous multiquantal release. AMPA-R function in this motor pathway thus appears to be independent of previous NMDA-R activation, unlike other regions of the developing nervous system, ensuring a greater reliability for embryonic excitatory transmission. Early spontaneous excitatory activity is specialized to promote AMPA-R-mediated synaptic Ca(2+) influx, which likely has significant roles in neuronal development.
Figures







References
-
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are colocalized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
-
- Bekkers JM, Stevens CF. Quantal analysis of EPSCs recorded from small numbers of synapses in hippocampal cultures. J. Neurophysiol. 1995;73:1145–1156. - PubMed
-
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA, and AMPA receptors: a developmentally regulated “menage a trois.”. Trends Neurosci. 1997;20:523–529. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
