The representation of illusory and real contours in human cortical visual areas revealed by functional magnetic resonance imaging
- PMID: 10493756
- PMCID: PMC6783043
- DOI: 10.1523/JNEUROSCI.19-19-08560.1999
The representation of illusory and real contours in human cortical visual areas revealed by functional magnetic resonance imaging
Abstract
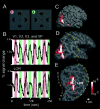
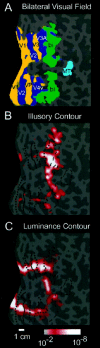
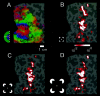
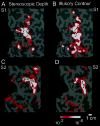
Illusory contours (perceived edges that exist in the absence of local stimulus borders) demonstrate that perception is an active process, creating features not present in the light patterns striking the retina. Illusory contours are thought to be processed using mechanisms that partially overlap with those of "real" contours, but questions about the neural substrate of these percepts remain. Here, we employed functional magnetic resonance imaging to obtain physiological signals from human visual cortex while subjects viewed different types of contours, both real and illusory. We sampled these signals independently from nine visual areas, each defined by retinotopic or other independent criteria. Using both within- and across-subject analysis, we found evidence for overlapping sites of processing; most areas responded to most types of contours. However, there were distinctive differences in the strength of activity across areas and contour types. Two types of illusory contours differed in the strength of activation of the retinotopic areas, but both types activated crudely retinotopic visual areas, including V3A, V4v, V7, and V8, bilaterally. The extent of activation was largely invariant across a range of stimulus sizes that produce illusory contours perceptually, but it was related to the spatial frequency of displaced-grating stimuli. Finally, there was a striking similarity in the pattern of results for the illusory contour-defined shape and a similar shape defined by stereoscopic depth. These and other results suggest a role in surface perception for this lateral occipital region that includes V3A, V4v, V7, and V8.
Figures




Similar articles
-
Early processing in the human lateral occipital complex is highly responsive to illusory contours but not to salient regions.Eur J Neurosci. 2009 Nov;30(10):2018-28. doi: 10.1111/j.1460-9568.2009.06981.x. Epub 2009 Nov 6. Eur J Neurosci. 2009. PMID: 19895562 Free PMC article.
-
Neuronal correlates of real and illusory contour perception: functional anatomy with PET.Eur J Neurosci. 1999 Nov;11(11):4024-36. doi: 10.1046/j.1460-9568.1999.00805.x. Eur J Neurosci. 1999. PMID: 10583491
-
The Poggendorff illusion driven by real and illusory contour: Behavioral and neural mechanisms.Neuropsychologia. 2016 May;85:24-34. doi: 10.1016/j.neuropsychologia.2016.03.005. Epub 2016 Mar 5. Neuropsychologia. 2016. PMID: 26956926
-
Human cortical areas underlying the perception of optic flow: brain imaging studies.Int Rev Neurobiol. 2000;44:269-92. doi: 10.1016/s0074-7742(08)60746-1. Int Rev Neurobiol. 2000. PMID: 10605650 Review.
-
Illusions in the spatial sense of the eye: geometrical-optical illusions and the neural representation of space.Vision Res. 2008 Sep;48(20):2128-42. doi: 10.1016/j.visres.2008.05.016. Epub 2008 Jul 7. Vision Res. 2008. PMID: 18606433 Review.
Cited by
-
Amodal completion instead of predictive coding can explain activity suppression of early visual cortex during illusory shape perception.J Vis. 2021 May 3;21(5):13. doi: 10.1167/jov.21.5.13. J Vis. 2021. PMID: 33988675 Free PMC article.
-
Influence of parallel and orthogonal real lines on illusory contour perception.J Neurophysiol. 2010 Jan;103(1):55-64. doi: 10.1152/jn.00001.2009. Epub 2009 Oct 28. J Neurophysiol. 2010. PMID: 19864444 Free PMC article.
-
Retinotopic activation in response to subjective contours in primary visual cortex.Front Hum Neurosci. 2008 Apr 3;2:2. doi: 10.3389/neuro.09.002.2008. eCollection 2008. Front Hum Neurosci. 2008. PMID: 18958203 Free PMC article.
-
Orientation-cue invariant population responses to contrast-modulated and phase-reversed contour stimuli in macaque V1 and V2.PLoS One. 2014 Sep 4;9(9):e106753. doi: 10.1371/journal.pone.0106753. eCollection 2014. PLoS One. 2014. PMID: 25188576 Free PMC article.
-
Early processing in the human lateral occipital complex is highly responsive to illusory contours but not to salient regions.Eur J Neurosci. 2009 Nov;30(10):2018-28. doi: 10.1111/j.1460-9568.2009.06981.x. Epub 2009 Nov 6. Eur J Neurosci. 2009. PMID: 19895562 Free PMC article.
References
-
- Collins LD, Neelin P, Peters TM, Evans AC. Automatic 3D inter-subject registration of MR volumetric data in standardized Talairach space. J Comp Assist Tomog. 1994;18:192–205. - PubMed
-
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction. J Cognit Neurosci. 1999;5:162–176. - PubMed
-
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. - PubMed
-
- DeYoe EA, Carmen GJ, Bandettini PA, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Functional magnetic resonance imaging (fMRI) of the human brain. J Neurosci Methods. 1994;54:171–187. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
