GABA(B) receptors inhibit mechanosensitivity of primary afferent endings
- PMID: 10493759
- PMCID: PMC6783028
- DOI: 10.1523/JNEUROSCI.19-19-08597.1999
GABA(B) receptors inhibit mechanosensitivity of primary afferent endings
Abstract
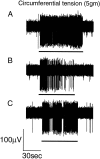
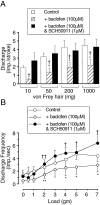
The modulatory effects of baclofen on the sensitivity of peripheral afferent endings to mechanical stimulation were investigated using an in vitro ferret gastroesophageal vagal afferent preparation. Changes in sensitivity of three types of gastroesophageal vagal afferent endings previously categorized as mucosal, tension, and tension-mucosal (TM) receptors according to their mechanoreceptive field characteristics were investigated. Baclofen (30-200 microM) dose dependently reduced responses of mucosal afferents to mucosal stroking with calibrated von Frey hairs (10-1000 mg). This was reversed by the GABA(B) receptor antagonist SCH50911 (1 microM). TM afferent responses to mucosal stroking (10-1000 mg) were unaffected by baclofen (30-200 microM). However, baclofen (30-200 microM) significantly inhibited the response of 11 of 18 TM afferents to circumferential tension. This was reversed by SCH50911 (1 microM). Baclofen (100 and 200 microM) significantly inhibited the response of all tension receptor afferents to circumferential tension in the lower range (1-3 gm) but not in the higher range (4-7 gm). This inhibition was reversed by SCH50911 (1 microM; n = 3). This study provides the first direct evidence for the inhibitory modulation of peripheral mechanosensory endings by the G-protein-coupled GABA(B) receptor. Inhibition was dose-dependent, pharmacologically reversible, and selective to certain aspects of mechanosensitivity. These findings have important relevance to strategies for selective reduction of sensory input to the CNS at a peripheral site.
Figures




Similar articles
-
Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents.Am J Physiol Gastrointest Liver Physiol. 2007 May;292(5):G1376-84. doi: 10.1152/ajpgi.00536.2006. Epub 2007 Feb 8. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17290011
-
Inhibition of mechanosensitivity in visceral primary afferents by GABAB receptors involves calcium and potassium channels.Neuroscience. 2006;137(2):627-36. doi: 10.1016/j.neuroscience.2005.09.016. Epub 2005 Nov 14. Neuroscience. 2006. PMID: 16289839
-
GABA(B)R expressed on vagal afferent neurones inhibit gastric mechanosensitivity in ferret proximal stomach.Am J Physiol Gastrointest Liver Physiol. 2001 Dec;281(6):G1494-501. doi: 10.1152/ajpgi.2001.281.6.G1494. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11705755
-
Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5.Am J Physiol Gastrointest Liver Physiol. 2007 Feb;292(2):G501-11. doi: 10.1152/ajpgi.00353.2006. Epub 2006 Oct 19. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17053158
-
Receptors and transmission in the brain-gut axis: potential for novel therapies. IV. GABA(B) receptors in the brain-gastroesophageal axis.Am J Physiol Gastrointest Liver Physiol. 2001 Aug;281(2):G311-5. doi: 10.1152/ajpgi.2001.281.2.G311. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11447009 Review.
Cited by
-
Gastroesophageal reflux disease and baclofen: is there a light at the end of the tunnel?Curr Gastroenterol Rep. 2004 Jun;6(3):213-9. doi: 10.1007/s11894-004-0010-9. Curr Gastroenterol Rep. 2004. PMID: 15128488 Review.
-
Neural mechanisms and delayed gastric emptying of liquid induced through acute myocardial infarction in rats.Arq Bras Cardiol. 2015 Feb;104(2):144-51. doi: 10.5935/abc.20140190. Epub 2014 Dec 9. Arq Bras Cardiol. 2015. PMID: 25494017 Free PMC article. English, Portuguese.
-
Influence of GABA-B Agonist Baclofen on Capsaicin-Induced Excitation of Secondary Peristalsis in Humans.Clin Transl Gastroenterol. 2017 Oct 5;8(10):e120. doi: 10.1038/ctg.2017.46. Clin Transl Gastroenterol. 2017. PMID: 28981081 Free PMC article.
-
Involvement of galanin receptors 1 and 2 in the modulation of mouse vagal afferent mechanosensitivity.J Physiol. 2007 Sep 1;583(Pt 2):675-84. doi: 10.1113/jphysiol.2007.135939. Epub 2007 Jul 12. J Physiol. 2007. PMID: 17627995 Free PMC article.
-
Control of transient lower oesophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in patients with gastro-oesophageal reflux disease.Gut. 2002 Jan;50(1):19-24. doi: 10.1136/gut.50.1.19. Gut. 2002. PMID: 11772961 Free PMC article. Clinical Trial.
References
-
- Awapara J, Landua AJ, Fuerst R, Seale B. Free γ-aminobutyric acid in brain. J Biol Chem. 1950;187:35–39. - PubMed
-
- Bakhle YS, Bell C. Neurokinin A and substance P vary independently in different regions of rat sensory neurons. Neuropeptides. 1995;28:237–241. - PubMed
-
- Berthoud H-R, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
