Cholinergic induction of theta-frequency oscillations in hippocampal inhibitory interneurons and pacing of pyramidal cell firing
- PMID: 10493764
- PMCID: PMC6783040
- DOI: 10.1523/JNEUROSCI.19-19-08637.1999
Cholinergic induction of theta-frequency oscillations in hippocampal inhibitory interneurons and pacing of pyramidal cell firing
Abstract
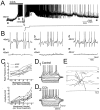
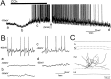
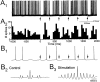
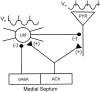
Cholinergic and GABAergic medial septal afferents contribute to hippocampal theta activity in part by actions on local interneurons. Interneurons near the border between stratum radiatum and stratum lacunosum-moleculare (LM) display intrinsic membrane potential oscillations at theta frequency when depolarized near threshold. First, whole-cell current-clamp recordings in rat hippocampal slices were used to examine effects of the cholinergic agonist carbachol on biocytin-labeled LM interneurons. At resting membrane potential, cells were depolarized by bath application of 25 microM carbachol, and the depolarization was sufficient to induce membrane potential oscillations (2.4 +/- 0.2 mV) that paced cell firing. Carbachol also depolarized LM interneurons in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione, (+/-)-2-amino-5-phosphonopentanoic acid, and bicuculline, indicating that cholinergic depolarization of LM cells does not depend on ionotropic glutamate or GABA(A) synaptic transmission in local circuits. Atropine blocked the depolarization, indicating that muscarinic receptors were involved. Minimal stimulation applied to visually identified LM interneurons was then used to determine if spontaneous activity in CA1 pyramidal cells can be paced by rhythmic inhibition generated by LM cells at theta frequency. Inhibitory postsynaptic potentials evoked in pyramidal cells by single minimal stimulations were followed by rebound depolarizations and action potentials. When trains of minimal stimulation were delivered, membrane potential oscillations of depolarized pyramidal cells followed the stimulation frequency. Minimal stimulation led pyramidal cell firing with an average phase of 177 degrees. Thus, muscarinic induction of theta-frequency membrane potential oscillations in LM interneurons may contribute to the generation of rhythmic inhibition that paces intrinsically generated theta activity in CA1 pyramidal cells.
Figures


Similar articles
-
Membrane properties and synaptic currents evoked in CA1 interneuron subtypes in rat hippocampal slices.J Neurophysiol. 1996 Jul;76(1):1-16. doi: 10.1152/jn.1996.76.1.1. J Neurophysiol. 1996. PMID: 8836204
-
Membrane properties and synaptic responses of interneurons located near the stratum lacunosum-moleculare/radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices.J Neurophysiol. 1994 Jun;71(6):2217-35. doi: 10.1152/jn.1994.71.6.2217. J Neurophysiol. 1994. PMID: 7931512
-
Intrinsic theta-frequency membrane potential oscillations in hippocampal CA1 interneurons of stratum lacunosum-moleculare.J Neurophysiol. 1999 Mar;81(3):1296-307. doi: 10.1152/jn.1999.81.3.1296. J Neurophysiol. 1999. PMID: 10085356
-
Generation of theta and gamma rhythms in the hippocampus.Neurosci Biobehav Rev. 1998 Mar;22(2):275-90. doi: 10.1016/s0149-7634(97)00014-6. Neurosci Biobehav Rev. 1998. PMID: 9579318 Review.
-
Inhibitory Postsynaptic Potentials Participate in Intracellular and Extracellular Theta Rhythms in the Hippocampus: A Personal Narrative.Hippocampus. 2025 Jan;35(1):e23660. doi: 10.1002/hipo.23660. Hippocampus. 2025. PMID: 39670347 Review.
Cited by
-
Optogenetic release of ACh induces rhythmic bursts of perisomatic IPSCs in hippocampus.PLoS One. 2011;6(11):e27691. doi: 10.1371/journal.pone.0027691. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22110723 Free PMC article.
-
Altered GABAA,slow inhibition and network oscillations in mice lacking the GABAA receptor beta3 subunit.J Neurophysiol. 2009 Dec;102(6):3643-55. doi: 10.1152/jn.00651.2009. Epub 2009 Oct 21. J Neurophysiol. 2009. PMID: 19846622 Free PMC article.
-
Networks of interneurons with fast and slow gamma-aminobutyric acid type A (GABAA) kinetics provide substrate for mixed gamma-theta rhythm.Proc Natl Acad Sci U S A. 2000 Jul 5;97(14):8128-33. doi: 10.1073/pnas.100124097. Proc Natl Acad Sci U S A. 2000. PMID: 10869419 Free PMC article.
-
Target selectivity of septal cholinergic neurons in the medial and lateral entorhinal cortex.Proc Natl Acad Sci U S A. 2018 Mar 13;115(11):E2644-E2652. doi: 10.1073/pnas.1716531115. Epub 2018 Feb 27. Proc Natl Acad Sci U S A. 2018. PMID: 29487212 Free PMC article.
-
Simple, biologically-constrained CA1 pyramidal cell models using an intact, whole hippocampus context.F1000Res. 2014 May 9;3:104. doi: 10.12688/f1000research.3894.1. eCollection 2014. F1000Res. 2014. PMID: 25383182 Free PMC article.
References
-
- Atzori M. Pyramidal cells and stratum lacunosum-moleculare interneurons in the CA1 hippocampal region share a GABAergic spontaneous input. Hippocampus. 1996;6:72–78. - PubMed
-
- Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J Neurophysiol. 1993;69:626–629. - PubMed
-
- Bland BH, Colom LV. Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Prog Neurobiol. 1993;41:157–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
