Binding of Actinobacillus pleuropneumoniae lipopolysaccharides to glycosphingolipids evaluated by thin-layer chromatography
- PMID: 10496867
- PMCID: PMC96842
- DOI: 10.1128/IAI.67.10.4983-4987.1999
Binding of Actinobacillus pleuropneumoniae lipopolysaccharides to glycosphingolipids evaluated by thin-layer chromatography
Abstract
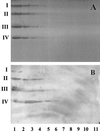
The binding profile of Actinobacillus pleuropneumoniae serotypes 1 and 2 to various glycosphingolipids was evaluated by using thin-layer chromatogram overlay. A. pleuropneumoniae whole cells recognized glucosylceramide (Glcbeta1Cer), galactosylceramide (Galbeta1Cer) with hydroxy and nonhydroxy fatty acids, sulfatide (SO(3)-3Galbeta1Cer), lactosylceramide (Galbeta1-4Glcbeta1Cer), gangliotriaosylceramide GgO3 (GalNAcbeta1-4Galbeta1-4Glcbeta1Cer), and gangliotetraosylceramide GgO4 (Galbeta1-3GalNAcbeta1-4Galbeta1-4Glcbeta1Cer) glycosphingolipids. We observed no binding to globoseries, globotriaosylceramide Gb3, globoside Gb4, or Forssman Gb5 glycosphingolipids or to gangliosides GM1, GM2, GM3, GD1a, GD1b, GD3, and GT1b. The A. pleuropneumoniae strains tested also failed to detect phosphatidylethanolamine or ceramide. Interestingly, extracted lipopolysaccharide (LPS) of serotype 1 and serotype 2 as well as detoxified LPS of serotype 1 showed binding patterns similar to that of whole bacterial cells. Binding to GlcCer, GalCer, sulfatide, and LacCer, but not to GgO3 and GgO4 glycosphingolipids, was inhibited after incubation of the bacteria with monoclonal antibodies against LPS O antigen. These findings indicate the involvement of LPS in recognition of three groups of glycosphingolipids: (i) GlcCer and LacCer, where glucose is probably an important saccharide sequence required for LPS binding; (ii) GalCer and sulfatide glycosphingolipids, where the sulfate group is part of the binding epitope of the isoreceptor; and (iii) GgO3 and GgO4, where GalNacbeta1-4Gal disaccharide represents the minimal common binding epitope. Taken together, our results indicate that A. pleuropneumoniae LPS recognize various saccharide sequences found in different glycosphingolipids, which probably represents a strong virulence attribute.
Figures


References
-
- Bock K, Breimer M E, Bringnole A, Hansson G C, Karlsson K-A, Larson G, Leffler H, Samuelsson B E, Strömberg N, Svanborg Edén C, Thurin J. Specificity of binding of a strain of uropathogenic Escherichia coli to Galα1-4Gal-containing glycosphingolipids. J Biol Chem. 1985;260:8545–8551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

