Cryptosporidium parvum apical complex glycoprotein CSL contains a sporozoite ligand for intestinal epithelial cells
- PMID: 10496907
- PMCID: PMC96882
- DOI: 10.1128/IAI.67.10.5282-5291.1999
Cryptosporidium parvum apical complex glycoprotein CSL contains a sporozoite ligand for intestinal epithelial cells
Abstract
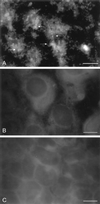
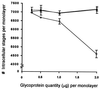
Cryptosporidiosis, caused by the apicomplexan parasite Cryptosporidium parvum, has become a well-recognized diarrheal disease of humans and other mammals throughout the world. No approved parasite-specific drugs, vaccines, or immunotherapies for control of the disease are currently available, although passive immunization with C. parvum-specific antibodies has some efficacy in immunocompromised and neonatal hosts. We previously reported that CSL, an approximately 1,300-kDa conserved apical glycoprotein of C. parvum sporozoites and merozoites, is the antigenic species mechanistically bound by neutralizing monoclonal antibody 3E2 which elicits the circumsporozoite precipitate (CSP)-like reaction and passively protects against C. parvum infection in vivo. These findings indicated that CSL has a functional role in sporozoite infectivity. Here we report that CSL has properties consistent with being a sporozoite ligand for intestinal epithelial cells. For these studies, native CSL was isolated from whole sporozoites by isoelectric focusing (IEF) following observations that the approximately 1,300-kDa region containing CSL as seen by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was comprised of approximately 15 molecular species (pI 3 to 10) when examined by two-dimensional (2-D) electrophoresis and silver staining. A subset of six approximately 1,300-kDa species (pI 4.0 to 6.5) was specifically recognized by 3E2 in 2-D Western immunoblots of IEF-isolated CSL. Isolated native CSL bound specifically and with high affinity to permissive human intestinal epithelial Caco-2 cells in a dose-dependent, saturable, and self-displaceable manner. Further, CSL specifically bound to the surface of live Caco-2 cells inhibited sporozoite attachment and invasion. In addition, sporozoites having released CSL after incubation with 3E2 and occurrence of the CSP-like reaction did not attach to and invade Caco-2 cells. These findings indicate that CSL contains a sporozoite ligand which facilitates attachment to and invasion of Caco-2 cells and, further, that ligand function may be disrupted by CSL-reactive monoclonal antibody. We conclude that CSL is a rational target for passive or active immunization against cryptosporidiosis.
Figures






References
-
- Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. - PubMed
-
- Barnes D A, Bonnin A, Huang J X, Gousset L, Wu J, Gut J, Doyle P, Dubremetz J F, Ward H, Petersen C. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol Biochem Parasitol. 1998;96:93–110. - PubMed
-
- Blagburn B L, Soave R. Prophylaxis and chemotherapy. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 111–123.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

