Intrinsic cone adaptation modulates feedback efficiency from horizontal cells to cones
- PMID: 10498670
- PMCID: PMC2229471
- DOI: 10.1085/jgp.114.4.511
Intrinsic cone adaptation modulates feedback efficiency from horizontal cells to cones
Abstract
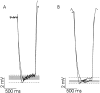
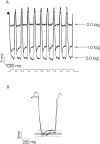
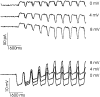
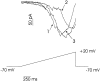
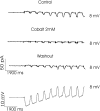
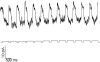
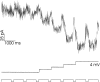
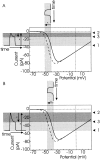
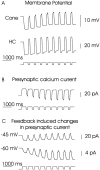
Processing of visual stimuli by the retina changes strongly during light/dark adaptation. These changes are due to both local photoreceptor-based processes and to changes in the retinal network. The feedback pathway from horizontal cells to cones is known to be one of the pathways that is modulated strongly during adaptation. Although this phenomenon is well described, the mechanism for this change is poorly characterized. The aim of this paper is to describe the mechanism for the increase in efficiency of the feedback synapse from horizontal cells to cones. We show that a train of flashes can increase the feedback response from the horizontal cells, as measured in the cones, up to threefold. This process has a time constant of approximately 3 s and can be attributed to processes intrinsic to the cones. It does not require dopamine, is not the result of changes in the kinetics of the cone light response and is not due to changes in horizontal cells themselves. During a flash train, cones adapt to the mean light intensity, resulting in a slight (4 mV) depolarization of the cones. The time constant of this depolarization is approximately 3 s. We will show that at this depolarized membrane potential, a light-induced change of the cone membrane potential induces a larger change in the calcium current than in the unadapted condition. Furthermore, we will show that negative feedback from horizontal cells to cones can modulate the calcium current more efficiently at this depolarized cone membrane potential. The change in horizontal cell response properties during the train of flashes can be fully attributed to these changes in the synaptic efficiency. Since feedback has major consequences for the dynamic, spatial, and spectral processing, the described mechanism might be very important to optimize the retina for ambient light conditions.
Figures




Similar articles
-
The open- and closed-loop gain-characteristics of the cone/horizontal cell synapse in goldfish retina.J Neurophysiol. 2000 Sep;84(3):1256-65. doi: 10.1152/jn.2000.84.3.1256. J Neurophysiol. 2000. PMID: 10980000
-
Modulation of horizontal cell receptive fields in the light adapted goldfish retina.Vision Res. 1996 Dec;36(24):3913-23. doi: 10.1016/s0042-6989(96)00185-x. Vision Res. 1996. PMID: 9068844
-
The nature of surround-induced depolarizing responses in goldfish cones.J Gen Physiol. 2000 Jan;115(1):3-16. doi: 10.1085/jgp.115.1.3. J Gen Physiol. 2000. PMID: 10613914 Free PMC article.
-
The involvement of glutamate-gated channels in negative feedback from horizontal cells to cones.Prog Brain Res. 2005;147:219-29. doi: 10.1016/S0079-6123(04)47017-4. Prog Brain Res. 2005. PMID: 15581709 Review.
-
The feedback pathway from horizontal cells to cones. A mini review with a look ahead.Vision Res. 1999 Jul;39(15):2449-68. doi: 10.1016/s0042-6989(99)00043-7. Vision Res. 1999. PMID: 10396615 Review.
Cited by
-
The dynamic characteristics of the feedback signal from horizontal cells to cones in the goldfish retina.J Physiol. 2001 Jul 15;534(Pt. 2):489-500. doi: 10.1111/j.1469-7793.2001.t01-1-00489.x. J Physiol. 2001. PMID: 11454966 Free PMC article.
-
Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels.PLoS Biol. 2011 Jul;9(7):e1001107. doi: 10.1371/journal.pbio.1001107. Epub 2011 Jul 19. PLoS Biol. 2011. PMID: 21811399 Free PMC article.
-
Enhancing the dark side: asymmetric gain of cone photoreceptors underpins their discrimination of visual scenes based on skewness.J Physiol. 2022 Jan;600(1):123-142. doi: 10.1113/JP282152. Epub 2021 Dec 8. J Physiol. 2022. PMID: 34783026 Free PMC article.
-
Lateral gain control in the outer retina leads to potentiation of center responses of retinal neurons.J Neurosci. 2009 May 13;29(19):6358-66. doi: 10.1523/JNEUROSCI.5834-08.2009. J Neurosci. 2009. PMID: 19439613 Free PMC article.
-
Chloride currents in cones modify feedback from horizontal cells to cones in goldfish retina.J Physiol. 2012 Nov 15;590(22):5581-95. doi: 10.1113/jphysiol.2012.240325. Epub 2012 Aug 13. J Physiol. 2012. PMID: 22890705 Free PMC article.
References
-
- Akopian A., McReynolds J.S., Weiler R. Short-term potentiation of off-responses in turtle horizontal cells. Brain Res. 1991;546:132–138. - PubMed
-
- Barnes S., Deschenes M.C. Contribution of Ca and Ca-activated Cl channels to regenerative depolarization and membrane bistability of cone photoreceptors. J. Neurophysiol. 1992;68:745–755. - PubMed
-
- Dong C.J., Werblin F.S. Dopamine modulation of GABAc receptor function in an isolated retinal neuron. J. Neurophysiol. 1994;71:1258–1260. - PubMed

