Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli
- PMID: 10498711
- PMCID: PMC103626
- DOI: 10.1128/JB.181.19.5993-6002.1999
Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli
Abstract
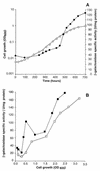
To get further information on bacterial surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli K-12, random insertion mutagenesis with Mu dX, a mini-Mu carrying the promoterless lacZ gene, was performed with an ompR234 adherent strain, and a simple screen was developed to assess changes in gene expression in biofilm cells versus planktonic cells. This screen revealed that major changes in the pattern of gene expression occur during biofilm development: the transcription of 38% of the genes was affected within biofilms. Different cell functions were more expressed in sessile bacteria: the OmpC porin, the high-affinity transport system of glycine betaine (encoded by the proU operon), the colanic acid exopolysaccharide (wca locus, formerly called cps), tripeptidase T (pepT), and the nickel high-affinity transport system (nikA). On the other hand, the syntheses of flagellin (fliC) and of a putative protein of 92 amino acids (f92) were both reduced in biofilms. Such a genetic reprogramming of gene expression in biofilms seems to result from changes in multiple environmental physicochemical conditions. In this work, we show that bacteria within biofilms encounter higher-osmolarity conditions, greater oxygen limitation, and higher cell density than in the liquid phase.
Figures

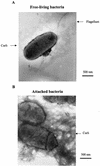

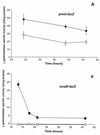
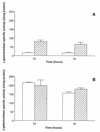
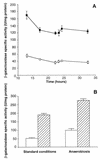
References
-
- Allison D G, Sutherland I W. The role of exopolysaccharides in adhesion of freshwater bacteria. J Gen Microbiol. 1987;133:1319–1327.
-
- Amann E, Brosius J. ’ATG’ for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40:183–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

