Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels
- PMID: 10498843
- PMCID: PMC1571612
- DOI: 10.1038/sj.bjp.0702775
Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels
Abstract
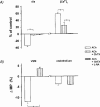
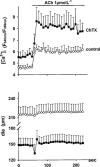
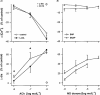
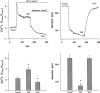
1. We hypothesized that nitric oxide (NO) and the endothelium-dependent hyperpolarizing factor (EDHF) may dilate microvessels by different cellular mechanisms, namely Ca2+-desensitization versus decrease in intracellular free calcium. 2. Effects of acetylcholine (ACh) and the NO donors sodium nitroprusside (SNP, 0.1 - 10 micromol l(-1)) and S-Nitroso-N-acetyl-D, L-penicillamine (SNAP, 0.01 - 10 micromol l-1) on intracellular calcium ([Ca2+]i, fura 2) and vascular diameter (videomicroscopy) were studied in isolated resistance arteries from hamster gracilis muscle (194+/-12 microm) pretreated with indomethacin and norepinephrine. Membrane potential changes were determined using 1, 3-dibutylbarbituric acid trimethineoxonol (DiBAC4(3)). 3. ACh (0.1 and 1 micromol l-1)-induced dilations were associated with a [Ca2+]i decrease (by 13+/-3 and 32+/-4%) and hyperpolarization of vascular smooth muscle (VSM, by 12+/-1% at 1 micromol l-1 ACh). Nomega-nitro-L-arginine (L-NA, 30 micromol l(-1)) partially inhibited the dilation but did not affect VSM [Ca2+]i decreases or hyperpolarization. In contrast, the KCa channel inhibitors tetrabutylammonium (TBA, 1 mmol l(-1)) and charybdotoxin (ChTX, 1 micromol l(-1)) abolished the ACh-induced [Ca2+]i decrease and the hyperpolarization in VSM while a significant dilation remained (25 and 40%). This remaining dilation was abolished by L-NA. ChTX did not affect [Ca2+]i increase and hyperpolarization in endothelial cells. SNP- or SNAP-induced dilations were not associated with decreases in VSM [Ca2+]i or hyperpolarization although minor transient decreases in VSM [Ca2+]i were observed at high concentrations. 4. These data suggest that ACh-induced dilations in microvessels are predominantly mediated by a factor different from NO and PGI2, presumably EDHF. EDHF exerts dilation by activation of KCa channels and a subsequent decrease in VSM [Ca2+]i, NO dilates the microvessels in a calcium-independent manner.
Figures





References
-
- ADEAGBO A.S., HENZEL M.K. Calcium-dependent phospholipase A2 mediates the production of endothelium-derived hyperpolarizing factor in perfused rat mesentery prearteriolar bed. J. Vascular Res. 1998;35:27–35. - PubMed
-
- ANDRIANTSITOHAINA R., LAGAUD G.J.L., ANDRE A., MULLER B., STOCLET J.C. Effects of cGMP on calcium handling in ATP-stimulated rat resistance arteries. Am. J. Physiol. 1995;268:H1223–H1231. - PubMed
-
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. - PubMed
-
- BRÄUNER T., HÜLSER D.F., STRASSER R.J. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim. Biophys. Acta. 1984;771:208–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

