Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity
- PMID: 10499920
- PMCID: PMC2195625
- DOI: 10.1084/jem.190.6.815
Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity
Abstract
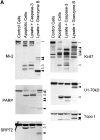
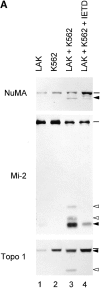
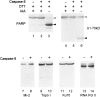
Systemic autoimmune diseases are a genetically complex, heterogeneous group of disorders in which the immune system targets a diverse but highly specific group of intracellular autoantigens. The molecules targeted are not unified by common structure, function, or distribution in control cells but become clustered and concentrated in surface blebs when cells undergo apoptosis. We show here that the majority of autoantigens targeted across the spectrum of human systemic autoimmune diseases are efficiently cleaved by granzyme B in vitro and during cytotoxic lymphocyte granule-induced death, generating unique fragments not observed during any other form of apoptosis. These molecules are not cleaved by caspase-8, although this protease has a very similar specificity to granzyme B. The granzyme B cleavage sites in autoantigens contain amino acids in the P(2) and P(3) positions that are preferred by granzyme B but are not tolerated by caspase-8. In contrast to autoantigens, nonautoantigens are either not cleaved by granzyme B or are cleaved to generate fragments identical to those formed in other forms of apoptosis. The striking ability of granzyme B to generate unique fragments is therefore an exclusive property of autoantigens and unifies the majority of molecules targeted in this spectrum of diseases. These results focus attention on the role of the cytotoxic lymphocyte granule-induced death pathway in the initiation and propagation of systemic autoimmunity.
Figures


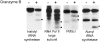





References
-
- Tan E.M. Antinuclear antibodiesdiagnostic markers for autoimmune diseases and probes for cell biology. Adv. Immunol. 1989;44:93–151. - PubMed
-
- Sercarz E.E., Lehmann P.V., Ametani A., Benichou G., Miller A., Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 1993;11:729–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

