A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein
- PMID: 10499923
- PMCID: PMC2195630
- DOI: 10.1084/jem.190.6.851
A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein
Abstract
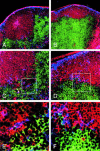
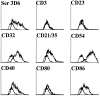
Dendritic cells (DCs) are known to activate naive T cells to become effective helper cells. In addition, recent evidence suggests that DCs may influence naive B cells during the initial priming of antibody responses. In this study, using three-color confocal microscopy and three-dimensional immunohistograms, we have observed that in the first few days after a primary immunization, cells with dendritic morphology progressively localize within primary B cell follicles. These cells were identified by their ability to bind a fusion protein consisting of the terminal cysteine-rich portion of the mouse mannose receptor and the Fc portion of human immunoglobulin (Ig)G1 (CR-Fc). In situ, these CR-Fc binding cells express major histocompatibility complex class II, sialoadhesin, and CD11c and are negative for other markers identifying the myeloid DC lineage, such as (CD11b), macrophages (F4/80), follicular DCs (FDC-M2), B cells (B220), and T cells (CD4). Using CR-Fc binding capacity and flow cytometry, the cells were purified from the draining lymph nodes of mice 24 h after immunization. When injected into naive mice, these cells were able to prime T cells as well as induce production of antigen-specific IgM and IgG1. Furthermore, they produced significantly more of the lymphocyte chemoattractant, macrophage inflammatory protein (MIP)-1alpha, than isolated interdigitating cells. Taken together, these results provide evidence that a subset of DCs enters primary follicles, armed with the capacity to attract and provide antigenic stimulation for T and B lymphocytes.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

