Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor
- PMID: 10499925
- PMCID: PMC2195627
- DOI: 10.1084/jem.190.6.875
Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor
Abstract
Idiopathic pulmonary alveolar proteinosis (I-PAP) is a rare disease of unknown etiology in which the alveoli fill with lipoproteinaceous material. We report here that I-PAP is an autoimmune disease with neutralizing antibody of immunoglobulin G isotype against granulocyte/macrophage colony-stimulating factor (GM-CSF). The antibody was found to be present in all specimens of bronchoalveolar lavage fluid obtained from 11 I-PAP patients but not in samples from 2 secondary PAP patients, 53 normal subjects, and 14 patients with other lung diseases. It specifically bound GM-CSF and neutralized bioactivity of the cytokine in vitro. The antibody was also found in sera from all I-PAP patients examined but not in sera from a secondary PAP patient or normal subjects, indicating that it exists systemically in I-PAP patients. As lack of GM-CSF signaling causes PAP in congenital cases and PAP-like disease in murine models, our findings strongly suggest that neutralization of GM-CSF bioactivity by the antibody causes dysfunction of alveolar macrophages, which results in reduced surfactant clearance.
Figures
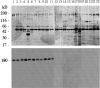
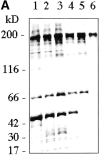




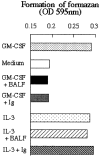

References
-
- van Golde L.M.G., Batenburg J.J., Robertson B. The pulmonary surfactant systembiochemical aspect and functional significance. Physiol. Rev. 1988;68:374–455. - PubMed
-
- Kuroki Y., Voelker D.R. Pulmonary surfactant protein. J. Biol. Chem. 1994;269:25943–25946. - PubMed
-
- Prakash U.B., Barham S.S., Carpenter H.A., Dines D.E., Marsh H.M. Pulmonary alveolar phospholipoproteinosisexperience with 34 cases and a review. Mayo Clin. Proc. 1987;62:499–518. - PubMed
-
- Ramirez R.J. Alveolar proteinosisimportance of pulmonary lavage. Am. Rev. Respir. Dis. 1971;103:666–678. - PubMed
-
- Alberti A., Luisetti M., Braschi A., Rodi G., Iotti G., Sella D., Poletti V., Benori V., Baritussio A. Bronchoalveolar lavage fluids composition in alveolar proteinosis. Early changes after therapeutic lavage. Am. J. Respir. Crit. Care Med. 1996;154:817–820. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

