A zinc-binding site in the largest subunit of DNA-dependent RNA polymerase is involved in enzyme assembly
- PMID: 10500100
- PMCID: PMC317019
- DOI: 10.1101/gad.13.18.2439
A zinc-binding site in the largest subunit of DNA-dependent RNA polymerase is involved in enzyme assembly
Abstract
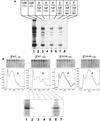
All multisubunit DNA-dependent RNA polymerases (RNAP) are zinc metalloenzymes, and at least two zinc atoms are present per enzyme molecule. RNAP residues involved in zinc binding and the functional role of zinc ions in the transcription mechanism or RNAP structure are unknown. Here, we locate four cysteine residues in the Escherichia coli RNAP largest subunit, beta', that coordinate one of the two zinc ions tightly associated with the enzyme. In the absence of zinc, or when zinc binding is prevented by mutation, the in vitro-assembled RNAP retains the proper subunit stoichiometry but is not functional. We demonstrate that zinc acts as a molecular chaperone, converting denatured beta' into a compact conformation that productively associates with other RNAP subunits. The beta' residues coordinating zinc are conserved throughout eubacteria and chloroplasts, but are absent from homologs from eukaryotes and archaea. Thus, the involvement of zinc in the RNAP assembly may be a unique feature of eubacterial-type enzymes.
Figures









References
-
- Allison LA, Moyle M, Shales M, Ingles CJ. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985;42:599–610. - PubMed
-
- Borukhov S, Goldfarb A. Recombinant Escherichia coli RNA polymerase: Purification of individually overexpressed subunits and in vitro assembly. Protein Expr Purif. 1993;4:503–511. - PubMed
-
- Borukhov S, Severinov K, Kashlev M, Lebedev A, Bass I, Rowland GC, Lim PP, Glass RE, Nikiforov V, Goldfarb A. Mapping of trypsin cleavage and antibody-binding sites and delineation of a dispensable domain in the beta subunit of Escherichia coli RNA polymerase. J Biol Chem. 1991;266:23921–23926. - PubMed
-
- Chatterji D, Wu FY-H. Selective substitution in vitro of an intrinsic zinc of Escherichia coli RNA polymerase with various divalent metals. Biochemistry. 1982;21:4651–4656. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
