The Escherichia coli sigma(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor
- PMID: 10500101
- PMCID: PMC317020
- DOI: 10.1101/gad.13.18.2449
The Escherichia coli sigma(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor
Abstract
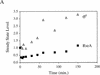
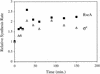
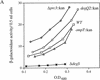
The activity of the stress-responsive sigma factor, sigma(E), is induced by the extracytoplasmic accumulation of misfolded or unfolded protein. The inner membrane protein RseA is the central regulatory molecule in this signal transduction cascade and acts as a sigma(E)-specific anti-sigma factor. Here we show that sigma(E) activity is primarily determined by the ratio of RseA to sigma(E). RseA is rapidly degraded in response to extracytoplasmic stress, leading to an increase in the free pool of sigma(E) and initiation of the stress response. We present evidence that the putative inner membrane serine protease, DegS, is responsible for this regulated degradation of RseA.
Figures









References
-
- Akiyama Y, Ito K. SecY protein, a membrane embedded secretion factor of E. coli, is cleaved by the OmpT protease in vitro. Biochem Biophys Res Commun. 1996;167:711–715. - PubMed
-
- Bartolomé B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. - PubMed
-
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
