The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study
- PMID: 10500111
- PMCID: PMC34229
- DOI: 10.1073/pnas.96.20.10976
The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study
Abstract
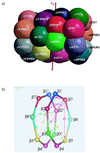
We present a biochemical and crystallographic characterization of active site mutants of the yeast 20S proteasome with the aim to characterize substrate cleavage specificity, subunit intermediate processing, and maturation. beta1(Pre3), beta2(Pup1), and beta5(Pre2) are responsible for the postacidic, tryptic, and chymotryptic activity, respectively. The maturation of active subunits is independent of the presence of other active subunits and occurs by intrasubunit autolysis. The propeptides of beta6(Pre7) and beta7(Pre4) are intermediately processed to their final forms by beta2(Pup1) in the wild-type enzyme and by beta5(Pre2) and beta1(Pre3) in the beta2(Pup1) inactive mutants. A role of the propeptide of beta1(Pre3) is to prevent acetylation and thereby inactivation. A gallery of proteasome mutants that contain active site residues in the context of the inactive subunits beta3(Pup3), beta6(Pre7), and beta7(Pre4) show that the presence of Gly-1, Thr1, Asp17, Lys33, Ser129, Asp166, and Ser169 is not sufficient to generate activity.
Figures



References
-
- Hilt W, Wolf D H. Trends Biochem Sci. 1996;21:96–102. - PubMed
-
- Hershko A, Ciechanover A. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Hochstrasser M. Annu Rev Genet. 1996;30:405–409. - PubMed
-
- Baumeister W, Walz J, Zühl F, Seemüller E. Cell. 1998;92:367–380. - PubMed
-
- Peters J M, Cejka Z, Harris R J, Kleinschmidt J A, Baumeister W. J Mol Biol. 1993;234:932–937. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

