Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for patched
- PMID: 10500113
- PMCID: PMC34231
- DOI: 10.1073/pnas.96.20.10992
Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for patched
Abstract
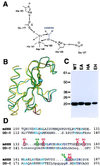
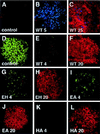
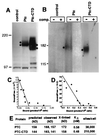
The amino-terminal signaling domain of the Sonic hedgehog secreted protein (Shh-N), which derives from the Shh precursor through an autoprocessing reaction mediated by the carboxyl-terminal domain, executes multiple functions in embryonic tissue patterning, including induction of ventral and suppression of dorsal cell types in the developing neural tube. An apparent catalytic site within Shh-N is suggested by structural homology to a bacterial carboxypeptidase. We demonstrate here that alteration of residues presumed to be critical for a hydrolytic activity does not cause a loss of inductive activity, thus ruling out catalysis by Shh-N as a requirement for signaling. We favor the alternative, that Shh-N functions primarily as a ligand for the putative receptor Patched (Ptc). This possibility is supported by new evidence for direct binding of Shh-N to Ptc and by a strong correlation between the affinity of Ptc-binding and the signaling potency of Shh-N protein variants carrying alterations of conserved residues in a particular region of the protein surface. These results together suggest that direct Shh-N binding to Ptc is a critical event in transduction of the Shh-N signal.
Figures
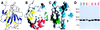
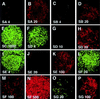
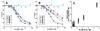
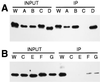
References
-
- Perrimon N. Cell. 1995;80:517–520. - PubMed
-
- Hammerschmidt M, Brook A, McMahon A P. Trends Genet. 1997;13:14–21. - PubMed
-
- Goodrich L V, Scott M P. Neuron. 1998;21:1243–1257. - PubMed
-
- Chiang C, Litingtung Y, Lee E, Young K, Corden J L, Westphal H, Beachy P. Nature (London) 1996;383:407–413. - PubMed
-
- Beachy P A, Cooper M K, Young K E, von Kessler D P, Park W J, Hall T M, Leahy D J, Porter J A. Cold Spring Harbor Symp Quant Biol. 1997;62:191–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

