Kinetic stability as a mechanism for protease longevity
- PMID: 10500115
- PMCID: PMC34233
- DOI: 10.1073/pnas.96.20.11008
Kinetic stability as a mechanism for protease longevity
Abstract
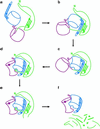
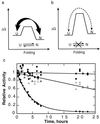
The folding of the extracellular serine protease, alpha-lytic protease (alphaLP; EC 3.4.21.12) reveals a novel mechanism for stability that appears to lead to a longer functional lifetime for the protease. For alphaLP, stability is based not on thermodynamics, but on kinetics. Whereas this has required the coevolution of a pro region to facilitate folding, the result has been the optimization of native-state properties independent of their consequences on thermodynamic stability. Structural and mutational data lead to a model for catalysis of folding in which the pro region binds to a conserved beta-hairpin in the alphaLP C-terminal domain, stabilizing the folding transition state and the native state. The pro region is then proteolytically degraded, leaving the active alphaLP trapped in a metastable conformation. This metastability appears to be a consequence of pressure to evolve properties of the native state, including a large, highly cooperative barrier to unfolding, and extreme rigidity, that reduce susceptibility to proteolytic degradation. In a test of survival under highly proteolytic conditions, homologous mammalian proteases that have not evolved kinetic stability are much more rapidly degraded than alphaLP. Kinetic stability as a means to longevity is likely to be a mechanism conserved among the majority of extracellular bacterial pro-proteases and may emerge as a general strategy for intracellular eukaryotic proteases subject to harsh conditions as well.
Figures


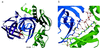
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

