Purification and DNA binding properties of the ataxia-telangiectasia gene product ATM
- PMID: 10500142
- PMCID: PMC17999
- DOI: 10.1073/pnas.96.20.11134
Purification and DNA binding properties of the ataxia-telangiectasia gene product ATM
Abstract
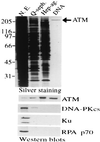
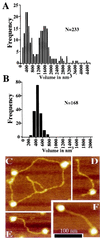
The human neurodegenerative and cancer predisposition condition ataxia-telangiectasia is characterized at the cellular level by radiosensitivity, chromosomal instability, and impaired induction of ionizing radiation-induced cell cycle checkpoint controls. Recent work has revealed that the gene defective in ataxia-telangiectasia, termed ATM, encodes an approximately 350-kDa polypeptide, ATM, that is a member of the phosphatidylinositol 3-kinase family. We show that ATM binds DNA and exploit this to purify ATM to near homogeneity. Atomic force microscopy reveals that ATM exists in two populations, with sizes consistent with monomeric and tetrameric states. Atomic force microscopy analyses also show that ATM binds preferentially to DNA ends. This property is similar to that displayed by the DNA-dependent protein kinase catalytic subunit, a phosphatidylinositol 3-kinase family member that functions in DNA damage detection in conjunction with the DNA end-binding protein Ku. Furthermore, purified ATM contains a kinase activity that phosphorylates serine-15 of p53 in a DNA-stimulated manner. These results provide a biochemical assay system for ATM, support genetic data indicating distinct roles for DNA-dependent protein kinase and ATM, and suggest how ATM may signal the presence of DNA damage to p53 and other downstream effectors.
Figures


References
-
- Hoekstra M F. Curr Opin Genet Dev. 1997;7:170–175. - PubMed
-
- Lavin M F, Shiloh Y. Annu Rev Immunol. 1997;15:177–202. - PubMed
-
- Rotman G, Shiloh Y. Hum Mol Genet. 1998;7:1555–1563. - PubMed
-
- Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J J. Cell. 1992;71:587–597. - PubMed
-
- Khanna K K, Lavin M F. Oncogene. 1993;12:3307–3312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

