Hepatic and intestinal transplantation at the University of Pittsburgh
- PMID: 10503105
- PMCID: PMC2956306
Hepatic and intestinal transplantation at the University of Pittsburgh
Figures








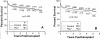




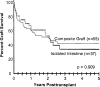




Similar articles
-
Clinical outcome of intestinal transplantation at the University of Miami.Transplant Proc. 1999 Feb-Mar;31(1-2):569-71. doi: 10.1016/s0041-1345(98)01558-9. Transplant Proc. 1999. PMID: 10083240 No abstract available.
-
Mycophenolate mofetil as primary and rescue therapy in intestinal transplantation.Transplant Proc. 1998 Sep;30(6):2677-9. doi: 10.1016/s0041-1345(98)00786-6. Transplant Proc. 1998. PMID: 9745545 No abstract available.
-
Status of liver and gastrointestinal transplantation at the University of Miami.Clin Transpl. 1996:187-201. Clin Transpl. 1996. PMID: 9286568
-
The current status of hepatic transplantation at the University of Pittsburgh.Clin Transpl. 1995:145-70. Clin Transpl. 1995. PMID: 8794262 Free PMC article. Review.
-
Pediatric transplantation.Am J Transplant. 2004;4 Suppl 9:54-71. doi: 10.1111/j.1600-6143.2004.00398.x. Am J Transplant. 2004. PMID: 15113355 Review.
Cited by
-
Immunomodulation for intestinal transplantation by allograft irradiation, adjunct donor bone marrow infusion, or both.Transplantation. 2000 Dec 15;70(11):1632-41. doi: 10.1097/00007890-200012150-00016. Transplantation. 2000. PMID: 11152226 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
