The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonuclear cells
- PMID: 10508024
- PMCID: PMC89500
- DOI: 10.1128/AAC.43.10.2457
The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonuclear cells
Abstract
HMR3647 is a semisynthetic representative of a new group of drugs, the ketolides, derived from erythromycin A. Since macrolides have been shown to accumulate in human polymorphonuclear cells (PMNs), we have investigated the ability of the molecule HMR3647 to enter human PMNs as well as other cell types, such as peripheral blood mononuclear cells and cell lines of hematopoietic and nonhematopoietic origin. In these experiments, HMR3647 was compared to erythromycin A, azithromycin, clarithromycin, and roxithromycin. Our results show that HMR3647 is specifically trapped in PMNs, where it is concentrated up to 300 times. In addition, it is poorly released by these cells, 80% of the compound remaining cell associated after 2 h in fresh medium. By contrast, it is poorly internalized and quickly released by the other cell types studied. This differs from the results obtained with the macrolide molecules, which behaved similarly in the different cells studied. In addition, subcellular fractionation of PMNs allowed us to identify the intracellular compartment where HMR3647 was trapped. In PMNs, more than 75% of the molecule was recovered in the azurophil granule fraction. Similarly, in NB4 cells differentiated into PMN-like cells, almost 60% of the molecules accumulated in the azurophil granule fraction. In addition, when HMR3647 was added to disrupted PMNs, 63% accumulated in the azurophil granules. Therefore, this study shows that the ketolide HMR3647 specifically accumulates in PMN azurophil granules, thus favoring its delivery to bacteria phagocytosed in these cells.
Figures

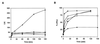





References
-
- Agouridas C, Benedetti Y, Denis A, Le Martret O, Chantot J-F. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Ketolides: a new distinct class of macrolide antibacterials. Synthesis and structural characteristics of RU 004, abstr. 157; p. 140.
-
- Barry A L, Brown S D, Fuchs P C. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Antipneumococcal activity of a ketolide (HMR3647) and seven related drugs in vivo, abstr. 107; p. 164.
-
- Borregaard N. Current concepts about neutrophil granule physiology. Curr Opin Hematol. 1996;3:11–18. - PubMed
-
- Borregaard N, Cowland J B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

