Possible role of xanthobaccins produced by Stenotrophomonas sp. strain SB-K88 in suppression of sugar beet damping-off disease
- PMID: 10508056
- PMCID: PMC91574
- DOI: 10.1128/AEM.65.10.4334-4339.1999
Possible role of xanthobaccins produced by Stenotrophomonas sp. strain SB-K88 in suppression of sugar beet damping-off disease
Abstract
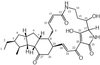
Three antifungal compounds, designated xanthobaccins A, B, and C, were isolated from the culture fluid of Stenotrophomonas sp. strain SB-K88, a rhizobacterium of sugar beet that suppresses damping-off disease. Production of xanthobaccin A in culture media was compared with the disease suppression activities of strain SB-K88 and less suppressive strains that were obtained by subculturing. Strain SB-K88 was applied to sugar beet seeds, and production of xanthobaccin A in the rhizosphere of seedlings was confirmed by using a test tube culture system under hydroponic culture conditions; 3 microg of xanthobaccin A was detected in the rhizosphere on a per-plant basis. Direct application of purified xanthobaccin A to seeds suppressed damping-off disease in soil naturally infested by Pythium spp. We suggest that xanthobaccin A produced by strain SB-K88 plays a key role in suppression of sugar beet damping-off disease.
Figures

References
-
- Abe H, Tamada T. A test tube culture system for multiplication of Polymyxa betae and necrotic yellow vein virus in rootlets of sugar beet. Proc Sugar Beet Res Assoc. 1987;29:34–38.
-
- Ahl P, Voisard C, Défago G. Iron-bound siderophores, cyanic acid, and antibiotics involved in suppression of Thielaviopsis basicola by a Pseudomonas fluorescens strain. J Phytopathol. 1986;116:121–134.
-
- Chen W, Hoitink H A J, Schmittenner A F. Factors affecting suppression of Pythium damping-off in container media amended with compost. Phytopathology. 1987;77:755–760.
-
- Dunne C, Moënne-Loccoz Y, McCarthy J, Higgins P, Powell J, Dowling D N, O’Gara F. Combining proteolytic and phloroglucinol-producing bacteria for improved biocontrol of Pythium-mediated damping-off of sugar beet. Plant Pathol. 1998;47:299–307.
-
- Giesler J L, Yuen Y G. Evaluation of Stenotrophomonas maltophilia strain C3 for biocontrol of brown patch disease. Crop Prot. 1998;17:509–513.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

