Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling
- PMID: 10508858
- PMCID: PMC2164973
- DOI: 10.1083/jcb.147.1.89
Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling
Abstract
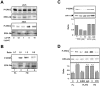
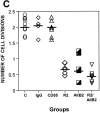
Mechanisms that regulate the transition of metastases from clinically undetectable and dormant to progressively growing are the least understood aspects of cancer biology. Here, we show that a large ( approximately 70%) reduction in the urokinase plasminogen activator receptor (uPAR) level in human carcinoma HEp3 cells, while not affecting their in vitro growth, induced a protracted state of tumor dormancy in vivo, with G(0)/G(1) arrest. We have now identified the mechanism responsible for the induction of dormancy. We found that uPA/uPAR proteins were physically associated with alpha5beta1, and that in cells with low uPAR the frequency of this association was significantly reduced, leading to a reduced avidity of alpha5beta1 and a lower adhesion of cells to the fibronectin (FN). Adhesion to FN resulted in a robust and persistent ERK1/2 activation and serum-independent growth stimulation of only uPAR-rich cells. Compared with uPAR-rich tumorigenic cells, the basal level of active extracellular regulated kinase (ERK) was four to sixfold reduced in uPAR-poor dormant cells and its stimulation by single chain uPA (scuPA) was weak and showed slow kinetics. The high basal level of active ERK in uPAR-rich cells could be strongly and rapidly stimulated by scuPA. Disruption of uPAR-alpha5beta1 complexes in uPAR-rich cells with antibodies or a peptide that disrupts uPAR-beta1 interactions, reduced the FN-dependent ERK1/2 activation. These results indicate that dormancy of low uPAR cells may be the consequence of insufficient uPA/uPAR/alpha5beta1 complexes, which cannot induce ERK1/2 activity above a threshold needed to sustain tumor growth in vivo. In support of this conclusion we found that treatment of uPAR-rich cells, which maintain high ERK activity in vivo, with reagents interfering with the uPAR/beta1 signal to ERK activation, mimic the in vivo dormancy induced by downregulation of uPAR.
Figures






References
-
- Andreasen P.A., Kjøller L., Christensen L., Duffy M.J. The urokinase-type plasminogen activator system in cancer metastasisa review. Int. J. Cancer. 1997;72:1–22. - PubMed
-
- Bazzoni G., Ma L., Blue M.L., Hemler M.E. Divalent cations and ligands induce conformational changes that are highly divergent among β1 integrins. J. Biol. Chem. 1998;273:6670–6678. - PubMed
-
- Chapman H.A. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr. Opin. Cell Biol. 1997;9:714–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

