Cell wall stress depolarizes cell growth via hyperactivation of RHO1
- PMID: 10508863
- PMCID: PMC2164985
- DOI: 10.1083/jcb.147.1.163
Cell wall stress depolarizes cell growth via hyperactivation of RHO1
Abstract
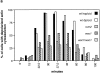
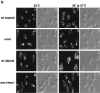
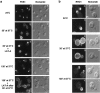
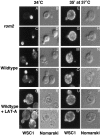
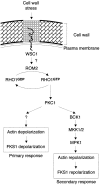
Cells sense and physiologically respond to environmental stress via signaling pathways. Saccharomyces cerevisiae cells respond to cell wall stress by transiently depolarizing the actin cytoskeleton. We report that cell wall stress also induces a transient depolarized distribution of the cell wall biosynthetic enzyme glucan synthase FKS1 and its regulatory subunit RHO1, possibly as a mechanism to repair general cell wall damage. The redistribution of FKS1 is dependent on the actin cytoskeleton. Depolarization of the actin cytoskeleton and FKS1 is mediated by the plasma membrane protein WSC1, the RHO1 GTPase switch, PKC1, and a yet-to-be defined PKC1 effector branch. WSC1 behaves like a signal transducer or a stress-specific actin landmark that both controls and responds to the actin cytoskeleton, similar to the bidirectional signaling between integrin receptors and the actin cytoskeleton in mammalian cells. The PKC1-activated mitogen-activated protein kinase cascade is not required for depolarization, but rather for repolarization of the actin cytoskeleton and FKS1. Thus, activated RHO1 can mediate both polarized and depolarized cell growth via the same effector, PKC1, suggesting that RHO1 may function as a rheostat rather than as a simple on-off switch.
Figures


References
-
- Alberts A.S., Bouquin N., Johnston L.H., Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 1998;273:8616–8622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

