Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin
- PMID: 10508864
- PMCID: PMC2164982
- DOI: 10.1083/jcb.147.1.175
Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin
Abstract
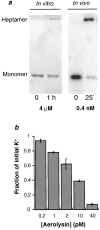
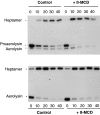
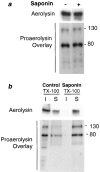
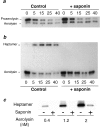
It has been proposed that the plasma membrane of many cell types contains cholesterol-sphingolipid-rich microdomains. Here, we analyze the role of these microdomains in promoting oligomerization of the bacterial pore-forming toxin aerolysin. Aerolysin binds to cells, via glycosyl phosphatidylinositol-anchored receptors, as a hydrophilic soluble protein that must polymerize into an amphipathic ring-like complex to form a pore. We first show that oligomerization can occur at >10(5)-fold lower toxin concentration at the surface of living cells than in solution. Our observations indicate that it is not merely the number of receptors on the target cell that is important for toxin sensitivity, but their ability to associate transiently with detergent resistant microdomains. Oligomerization appears to be promoted by the fact that the toxin bound to its glycosyl phosphatidylinositol-anchored receptors, can be recruited into these microdomains, which act as concentration devices.
Figures




References
-
- Abrami L., Fivaz M., Decroly E., Seidah N.G., François J., Thomas G., Leppla S., Buckley J.T., van der Goot F.G. The pore-forming toxin proaerolysin is processed by furin J. Biol. Chem 273 1998. 32656 32661a - PubMed
-
- Adam, G., and M. Delbrück. 1968. Structural Chemistry and Molecular Biology. A. Rich and N. Davidson, editors. W.H. Freeman & Co., NY.
-
- Bhakdi S., Bayley H., Valeva A., Walev I., Walker B., Kehoe M., Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysinprototypes of pore-forming bacterial cytolysins. Arch. Microbiol. 1996;165:73–79. - PubMed
-
- Bray D., Levin M.D., Morton-Firth C.J. Receptor clustering as a cellular mechanism to control sensitivity. Nature. 1998;393:85–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

